Surgery, Gastroenterology and Oncology

|
|
Introduction: The functional laparoscopic gastric bypass with fundectomy and gastric remnant exploration (LRYGBfse) has been described in attempt to overcome the intrinsic limitations of the LRYGB with promising results. However, the detailed learning curve related to this innovative procedure has not been assessed.
Materials and Methods: Retrospective multicenter study from December 2010 to January 2015. Data were prospectively collected from one surgeon experienced in laparoscopic surgery. The cumulative summation methodology (CUSUM) was applied to visualize the learning curve of LRYGBfse.
Results: Overall, 293 LRYGBfse were included. The majority were females (58%) and the median age of was 41.3 years (range 26-58). The median preoperative body mass index was 44.9 kg/m2 (range 36.4-50.3). The median operative time (OT) was 197 min (range 124–327). The 90-day morbidity rate was 1.32%. The CUSUM showed that the number of consecutive procedures needed to reach competency was 107 thus identifying two distinct phases of the curve. The comparative analysis did not show statistically significant differences for demographics, preoperative BMI, comorbidities, reoperation (1.86% vs. 0.53%; p=0.064) and overall complications (1.8% vs. 1.07%; p=0.47) between the two phases. OT (232 vs. 185 minutes; p=0.017) and hospital length of stay (6 vs. 4; p=0.041) were significantly reduced in the second phase.
Conclusions: The functional LRYGBfse seems feasible and safe even in the first phase of the learning curve. Our data seem to suggest that 107 procedures are required to gain LRYGBfse competence with a significant decrease of operative time and hospital length of stay.
INTRODUCTION
The laparoscopic Roux en-Y gastric bypass (LRYGB) is a worldwide performed operation for obesity and is considered by many the gold standard treatment because its exceptional outcomes in term of weight loss, comorbid resolution, and quality of life improvement (1-4). The challenging diagnosis and treatment of emerging diseases in the gastric remnant, duodenum, and common bile duct constitute an intrinsic limitation (5-7). Mastery of LRYGBP is reported to require a steep learning curve where operative time and morbidity rate may be augmented (8). Nowadays, most authors agree that the LRYGB learning curve involves 75-100 cases per surgeon (9-10).
The functional laparoscopic gastric bypass with fundectomy and gastric remnant exploration (LRYGBfse) has been described in attempt to overcome the intrinsic limitations of the LRYGB (11-13). The procedure consists in the creation of a small gastric pouch (about 20-30 cc) by resecting the gastric fundus. A polytetrafluoroethylene banding (ePTFE) is placed at the gastro-gastric communication (7 cm below thecardia) to divert the bolus through the alimentary limb with duodenal and jejunal functional exclusion. The endoscopic exploration of the remnant and duodenum is feasible via gentle endoscope advancement through the gastro-gastric communication. Previous studies reported the promising results of the LRYGBfse in term of weight-loss, comorbid resolution and quality of life improvement (14) however, the learning curve related with LRYGBfse has not been appraised yet.
This study aimed to evaluate surgeon learning curve for LRYGBfse using the cumulative summation methodology (CUSUM).
MATERIALS AND METHODS
Patient selection
Between December 2010 and January 2015, we conducted a prospective multicenter study on LRYGBfse. Data were entered into a dedicated dataset and reviewed retrospectively. All the procedures were performed by one senior surgeons (GL) experienced in laparoscopic surgery. All included patients met the National Institute consensus for bariatric surgery (15) and the Italian guidelines, according to the IFSO (International Federation for the Surgery of Obesity and Metabolic Disorders) guidelines, for surgery in the morbidly obese patient (http://www.sicob.it). All subjects provided written informed agreement to be part of the study. The study was approved by the local Research Ethics Committee. Primary aim was to assess one surgeon learning curve to attain competency with the LRYGBfse.
Operative time (OT) was defined as the time between skin incision and closure. Collected data: age, sex, preoperative weight (kg), body mass index (BMI), intraoperative complications, postoperative complications (90-day morbidity), and readmission rates (90 days). A complication was defined as any deviation from the normal postoperative course and ranked according to the Dindo-Clavien severity classification (16).
SURGICAL TECHNIQUE
Preoperative evaluation and surgical procedures have been described previously (11-12). Briefly, after the placement of a 36-Fr orogastric probe, fundectomy is completed with EndoGIA™ linear stapler firings (Medtronic, Minneapolis, MN, USA) to create a 20-30cc gastric pouch. A polytetrafluoroethylene banding (ePTFE) is placed at the gastro-gastric communication (7 cm below the cardia) and gently closed after bougie retraction. The bypass is completed by the creation of an antecolic Roux-en-Y 150 cm alimentary and 150 cm biliopancreatic limb. Linear side-to-side gastrojejunal and jejuno-jejunal anastomosis (45-mm EndoGIA™) are fashioned (fig. 1). Petersen’s defect is routinely closed.
Figure 1 - The functional laparoscopic gastric bypass with fundectomy and gastric remnant exploration (LRYGBfse) technique.

Cumulative sum analysis
The CUSUM technique was used for the learning curve quantitative assessment (17-18). The CUSUM is the running total of differences between the individual data points and the mean of all the data points (19-20). The CUSUM was used to evaluate the OT for all 293 cases. To compute the CUSUM, the cases were ordered chronologically. The CUSUM of the first case was the difference between the OT for the first case and the mean OT for all the cases. The CUSUM of the second case was the previous case’s CUSUM added to the difference between the OT for the second case and the mean OT for all the cases. The process was continued for all included patients. As in the study by Bokhari et al. (21), risk adjusted CUSUM was not performed because no deaths occurred in this series and the postoperative complication rate was low (n=4).
Statistical analysis
Continuous data are reported as median and range. Categorical data are reported using absolute and percentage frequencies. Chi-square or Mann-Whitney tests were performed as proper. Two-sided p values were calculated. P value equal or less than 0.05 was considered statistically significant. R version 3.2.2 software was used for statistical analysis (22).
RESULTS
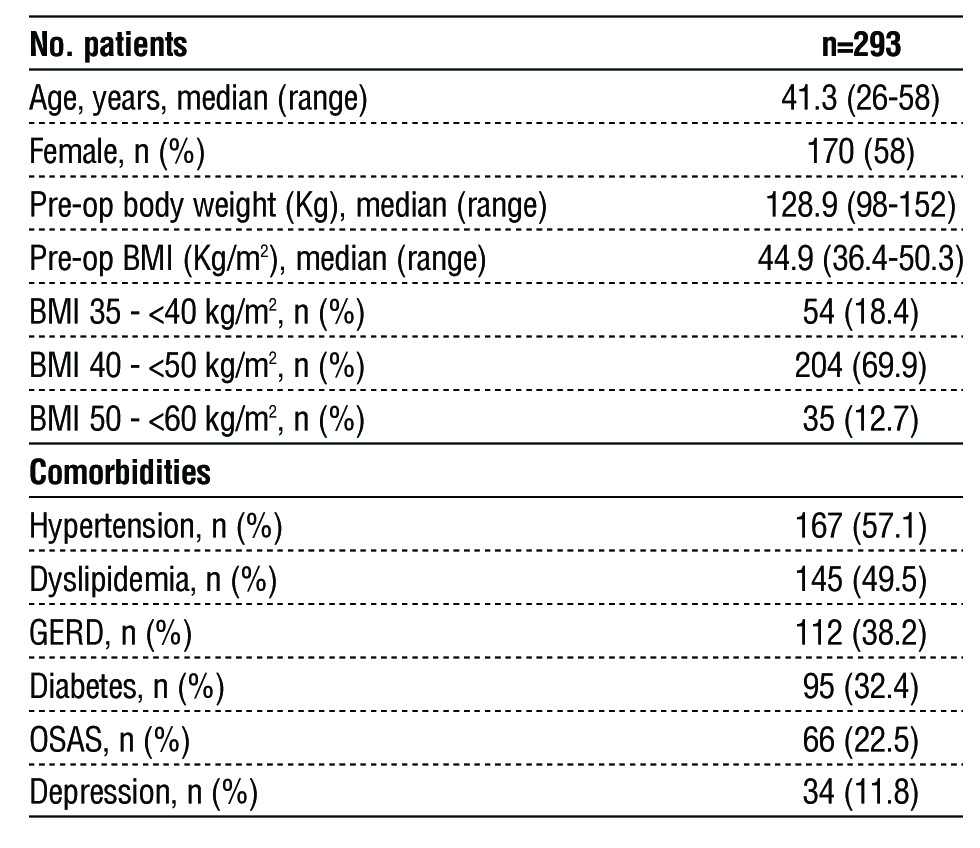
During the study period, 293 consecutive LRYGBfse were included. The patients were 170 women (58%) and the median age of was 41.3 years (range 26-58). The median preoperative BMI was 44.9 kg/m2 (range 36.4-50.3) and the median preoperative weight was 128.9 kg (range 98-152.1). Baseline demographics are presented in table 1.
Table 1 - Baseline patients’ demographics. Data are presented as median (IQR), and n (%). Legend. BMI: Body mass index; GERD gastroesophageal reflux disease; OSAS: Obstructive sleep apnea syndrome. Data are presented as median (range) for continuous variables and as number (percentage) for categorical variables
Perioperative results are summarized in table 2. The median OT was 197 min (range, 124–327). No intraoperative complications or conversions to open procedure occurred. The postoperative 90-day morbidity rate was 1.32% (n=4). In one patient the postoperative course was complicated by gastro-jejunal anastomotic bleeding successfully managed with endoscopic clipping (Grade IIIa). In three patients, a reoperation (Grade IIIb) was required because intra-abdominal bleeding, internal hernia and intestinal occlusion sustained by trocar site hernia. The median postoperative hospital length of stay was 4 days (range 2–12) and median intensive care unit length of stay was 1 (range 1–3). None of the patients required postoperative mechanical ventilator assistance. The overall mortality was 0% (95% CI 0.0–0.5%).
Table 2 - Perioperative outcomes. OT operative time; ICU intensive care unit; HLOS hospital length of stay. Data are presented as median (range) for continuous variables and as number (percentage) for categorical variables.

The raw OT data were plotted in chronological order (fig. 2). The CUSUM learning curve is depicted in fig. 3. The CUSUM-LRYGBfse showed that the number of operations required to reach competency and overcome the learning curve was 107. Comparison of parameters between the two phases identified by CUSUM analysis is described table 3. No differences were found in term of patient demographics comparing the two surgical phases. Postoperative complications (1.9% vs. 1.1; p=0.967) reoperation (1.9% vs. 0.5%; p=0.625) and 90-day hospital readmission (0.9% vs. 0.0%; p=0.879) were also comparable. OT (232 vs. 185 minutes; p=0.017) and HLOS (6 vs. 4 days; p=0.041) were significantly reduced between phase 1 vs. phase 2.
Figure 2 - Learning curve: operative time in minutes (Y axis) plotted against consecutive case number (X axis). The blue line represents the learning curve using loess fit with relative 95% confidence bands.

Figure 3 - Cumulative sum (CUSUM) for operative time (Y axis) plotted against consecutive case number (X axis). The blue line represents the CUSUM learning curve using loess fit with relative 95% confidence bands. The vertical red line indicates the turning point at which the surgeon transitions from phase 1 to phase 2 and overcome the OT-learning curve (n=107).
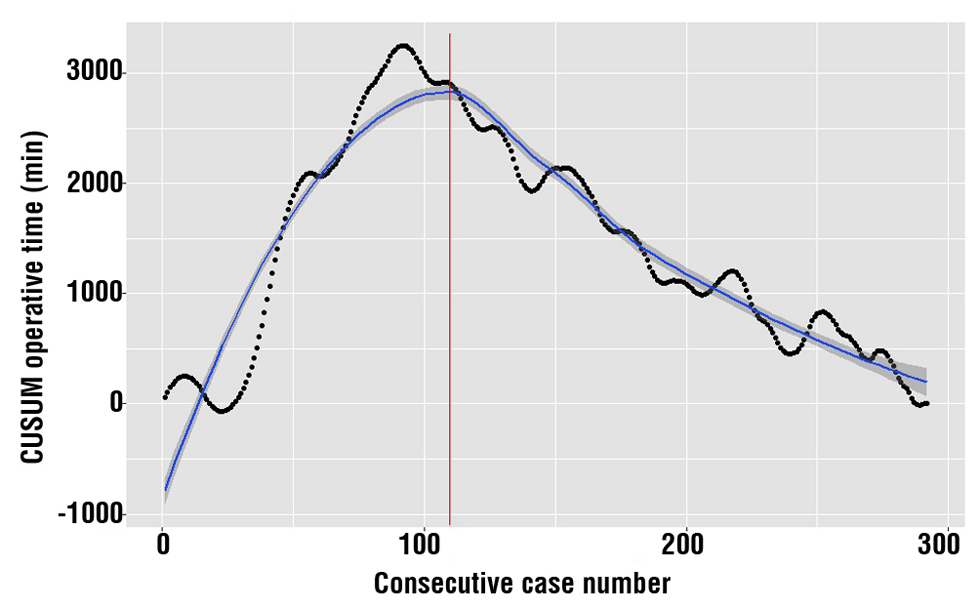
Table 3 - Comparisons between phases of patient characteristics and perioperative outcomes. Legend. BMI: Body mass index; GERD gastroesophageal reflux disease; OSAS: Obstructive sleep apnea syndrome. Data are presented as median (range) for continuous variables and as number (percentage) for categorical variables.

DISCUSSION
The functional LRYGBfse seems feasible and safe while the possibility to endoscopically access the gastric remnant is a major advantage over the standard LRYGB. Using operative time as referral outcome, CUSUM-LRYGBfse curve presents its inflection point at the 107th case and implies that 106 procedures are needed to gain competence with this surgical procedure.
The learning curve has been definite as the whole learning process until the individual gathers enough experience to reach a plateau (23-24). It can be assessed using several outcome measures (i.e. operative time, postoperative complications, blood loss, conversion to open, etc.) that can be divided in two clusters: patient outcome variables and surgical efficiency variables (25). Operative time and postoperative complications have an important role for each cluster and are denoted as main parameters for the definition of the bariatric learning curve (26).
LRYGB has been shown to be associated with a steep learning curve principally because the difficult procedural steps and advanced technical ability such as intracorporeal knot fashioning and suturing in a limited and constricted working space (27). To date, articles investigating the learning curve for LRYGB based their scrutiny on chronological cases split into predefined segments, with univariate analysis performed to compare means across groups. Specifically, as reported by a recent review, the LRYGB learning curve has been reported to be widely distributed ranging from 30 to 500 cases (26). Specifically, Shen et al. demonstrated that proficiency with considerable decrease in operative time and postoperative complications may be achieved with 30 cases (28). In contrast, Shiroka et al. and Jacobsen et al. found that 100 procedures are necessary to halve operative time (29-30) while El-Kadre et al. demonstrated that 500 procedures are necessary to significantly reduce the risk of postoperative adverse event (31). Notably, heterogeneity related to such a wide distribution is attributable to different patient and surgeon-related factors such as preoperative BMI, surgeon experience, training, and proficiency definition.
The cumulative-sum (CUSUM) method was introduced 50 years ago in the United Kingdom (UK) and has been proposed as an effective tool for analysis of the learning process (32). Initially used in industrial quality controls, the CUSUM-method was first used 10 years ago as a means of monitoring surgical performance (25). CUSUM is a tendency representation of a series of repeated procedures calibrate to the mean value. Despite its limitations, it has been suggested CUSUM is the best tool for quality control in health care clinical domains (18). This sensitive methodology is feasible as it permits one to work with an unknown, increasing sample size and seem to be more efficient than the rest of the medical quality tools detecting changes (19). In addition, CUSUM analysis defines experience as a continuous variable (33-34). In bariatric surgery, the CUSUM method has already been used to assess robotic Roux-en-Y gastric bypass (35) and laparoscopic sleeve gastrectomy (36) but no reports have been published describing the number of cases that a surgeon is required to have worked on train and become proficient with LRYGBfse. This new surgical technique has been defined with the intent to overwhelm the limitations of the standard operation without altering its results (10-11). In the present series the CUSUM curve shows its inflection point at the 107th case and implies that 106 procedures are needed to gain competency with LRYGBfse. Interestingly, this result seems similar to previous series reporting data for LRYGB. Specifically, Ballesta-Lopez et al. (37) and Jacobsen et al. (20) that reported a significant reduction in OT after the first 100 LRYGB while a 2012 review concluded that 105 cases are necessary to non-fellowship trained surgeons to complete LRYGB-learning curve (38-39).
The comparative analysis for phase 1 vs. phase 2 did not show statistically significant differences forpatient demographics, preoperative BMI and patients’ comorbidities. A higher complication rate is expected during the first phase of the surgeon learning curve with anastomotic leak being one of the most dreaded complications. In our series, we did not observe any anastomotic leak while postoperative complication rates were similar comparing the two learning phases (1.9% vs. 1.1%; p=0.967). Interestingly, from a clinical point of view, a trend toward reduction may be supposed. This is similar to Shikora et al. that described a trend toward reduced postoperative complications after the first 100 LRYGB (28). These data seem to further corroborate the feasibility and safety of LRYGBfse in the first phase of the learning curve in the hands of surgeons’ experiences in minimally invasive surgery. Furthermore, despite the result did not reach statistical significance, a trend toward reduced reoperation rate was observed (1.9% vs. 0.5%; p=0.625) between the two phases. Finally, similarly to what reported by Sovik et al. (40) in the setting of LRYGB, we observed a statistically significant decrease of HLOS (p=0.041).
The present study has some limitations. First, previous laparoscopic practice, training and mentoring might have an influence on learning curve. Therefore, these results may not be generally applicable. Second, no comparison was performed with the standard LRYGB. However, this was not the aim of the study. Third, as the number of complications was limited, a CUSUM-adjusted postoperative complications analysis was not feasible.
CONCLUSIONS
The functional LRYGBfse seems feasible and safe even in the first phase of the learning curve. Our data seem to suggest that, using operative time as referral outcome, 106 procedures are required to gain competence with LRYGBfse with a significant decrease of mean operative time and reduced hospital length of stay.
Conflict of Interest
The authors declare no competing interests.
Funding: None
Ethical statement
All procedures performed in studies involving human participants were in accordance with the standards of the local Ethics Committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from each individual participant included in the study.
REFERENCES
1.Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric surgery worldwide 2013. Obes Surg. 2015; 25(10):1822–32.
2.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724-37.
3.Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2011. Obes Surg. 2013;23:427–36.
4.Aiolfi A, Tornese S, Bonitta G, Rausa E, Micheletto G, Bona D. Roux-en-Y gastric bypass: systematic review and Bayesian network meta-analysis comparing open, laparoscopic, and robotic approach. Surg Obes Relat Dis. 2019;15(6):985-994.
5.Grimes KL, Maciel VH, Mata W, Arevalo G, Singh K, Arregui ME. Complications of laparoscopic transgastric ERCP in patients with Roux-en-Y gastric bypass. Surg Endosc. 2015;29(7):1753-9.
6.Aiolfi A, Asti E, Rausa E, Bernardi D, Bonitta G, Bonavina L. Trans-Gastric ERCP After Roux-en-Y Gastric Bypass: Systematic Review and Meta-Analysis. Obes Surg. 2018;28(9):2836-2843.
7.Tornese S, Aiolfi A, Bonitta G, Rausa E, Guerrazzi G, Bruni PG, et al. Remnant Gastric Cancer After Roux-en-Y Gastric Bypass: Narrative Review of the Literature. Obes Surg. 2019;29(8):2609-2613.
8.Yu SC, Clapp BL, Lee MJ, Albrecht WC, Scarborough TK, Wilson EB. Robotic assistance provides excellent outcomes during the learning curve for laparoscopic Roux-en-Y gastric bypass: results from 100 robotic-assisted gastric bypasses. Am J Surg. 2006;192(6):746-9.
9.Pournaras DJ, Jafferbhoy S, Titcomb DR, Humadi S, Edmond JR, Mahon D, et al. Three hundred laparoscopic Roux-en-Y gastric bypasses: managing the learning curve in higher risk patients. Obes Surg. 2010;20(3):290-4.
10.Schauer P, Ikramuddin S, Hamad G, Gourash W. The learning curve for laparoscopic Roux-en-Y gastric bypass is 100 cases. Surg Endosc. 2003;17(2):212-5.
11.Lesti G, Zappa MA, Lesti F, Bona D, Aiolfi A. Functional Laparoscopic Roux-en-Y Gastric Bypass with Fundectomy and Gastric Remnant Exploration (LRYGBfse)-a Video Vignette. Obes Surg. 2021;31(5): 2350-2352.
12.Lesti G, Aiolfi A, Mozzi E, Altorio F, Lattuada E, Lesti F, et al. Laparoscopic Gastric Bypass with Fundectomy and Gastric Remnant Exploration (LRYGBfse): Results at 5-Year Follow-up. Obes Surg. 2018;28(9):2626-2633.
13.Porta A, Aiolfi A, Musolino C, Antonini I, Zappa MA. Prospective Comparison and Quality of Life for Single-Incision and Conventional Laparoscopic Sleeve Gastrectomy in a Series of Morbidly Obese Patients. Obes Surg. 2017;27(3):681-687.
14.Lesti G, Bona D, Sozzi A, Lesti F, Bonitta G, Zappa MA, et al. Impact of Functional Laparoscopic Gastric Bypass with Fundectomy and Gastric Remnant Exploration (LRYGBfse) on Patients' Quality of Life: Trajectory and 5-Year Follow-up Result. Obes Surg. 2020;30(8): 3046-3053.
15.NIH conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med. 1991; 115(12):956-61.
16.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187-96.
17.Steiner SH, Cook RJ, Farewell VT. Monitoring paired binary surgical outcomes using cumulative sum charts. Stat Med. 199915;18(1): 69-86.
18.Novoa NM, Varela G. Monitoring surgical quality: the cumulative sum (CUSUM) approach. Mediastinum. 2020;4:4.
19.Sibanda T, Sibanda N. The CUSUM chart method as a tool for continuous monitoring of clinical outcomes using routinely collected data. BMC Med Res Methodol. 2007;7:46.
20.Aiolfi A, Bona D, Guerrazzi G, Bonitta G, Rausa E, Panizzo V, et al. Intracorporeal Versus Extracorporeal Anastomosis in Laparoscopic Right Colectomy: An Updated Systematic Review and Cumulative Meta-Analysis. J Laparoendosc Adv Surg Tech A. 2020;30(4):402-412.
21. Bokhari MB, Patel CB, Ramos-Valadez DI, Ragupathi M, Haas EM. Learning curve for robotic-assisted laparoscopic colorectal surgery. Surg Endosc. 2011;25(3):855-60.
22.R Core Team R A Lang Environ Stat Comput R Found Stat Comput Vienna, Austria. 2021. https://www.R-project.org/.
23.Valsamis EM, Chouari T, O'Dowd-Booth C, Rogers B, Ricketts D. Learning curves in surgery: variables, analysis and applications. Postgrad Med J. 2018 Sep;94(1115):525-530.
24.Graham LA, Hawn MT. Learning Curves and the Challenges of Adopting New Surgical Techniques. JAMA Netw Open. 2019 Oct 2;2(10):e1913569.
25.Tekkis PP, Senagore AJ, Delaney CP, Fazio VW. Evaluation of the learning curve in laparoscopic colorectal surgery: comparison of right sided and left-sided resections. Ann Surg. 2005;242(1):83-91.
26.Wehrtmann FS, de la Garza JR, Kowalewski KF, Schmidt MW, Müller K, Tapking C, et al. Learning Curves of Laparoscopic Roux-en-Y Gastric Bypass and Sleeve Gastrectomy in Bariatric Surgery: a Systematic Review and Introduction of a Standardization. Obes Surg. 2020;30(2):640-656.
27.Doumouras AG, Saleh F, Anvari S, Gmora S, Anvari M, Hong D. Mastery in Bariatric Surgery: The Long-term Surgeon Learning Curve of Roux-en-Y Gastric Bypass. Ann Surg. 2018;267(3):489-494.
28.Shen SC, Tsai CY, Liao CH, Liu YY, Yeh TS, Liu KH. Learning curve of laparoscopic Roux-en-Y gastric bypass in an Asian low-volume bariatric unit. Asian J Surg. 2018;41(2):170-175.
29.Shikora SA, Kim JJ, Tarnoff ME, Raskin E, Shore R. Laparoscopic Roux-en-Y gastric bypass - Results and learning curve of a high-volume academic program. Arch Surg. 2005;140(4):362-7.
30.Jacobsen HJ, Bergland A, Raeder J, Gislason HG. High-volume bariatric surgery in a single center: safety, quality, cost-efficacy and teaching aspects in 2,000 consecutive cases. Obes Surg. 2012; 22(1):158-66.
31.El-Kadre L, Tinoco AC, Tinoco RC, Aguiar L, Santos T. Overcoming the learning curve of laparoscopic Roux-en-Y gastric bypass: a 12-year experience. Surg Obes Relat Dis. 2013; 9(6):867-72.
32.Page ES. Continuous Inspection Scheme. Biometrika 1954;41:100-15.
33.Yap CH, Colson ME, Watters DA. Cumulative sum techniques for surgeons: a brief review. ANZ J Surg. 2007 Jul;77(7):583-6.
34.Wohl H. The cusum plot: its utility in the analysis of clinical data. N Engl J Med. 1977;296(18):1044-5.
35.Buchs NC, Pugin F, Bucher P, Hagen ME, Chassot G, Koutny-Fong P, et al. Learning curve for robot-assisted Roux-en-Y gastric bypass. Surg Endosc. 2012;26(4):1116-21.
36.Fantola G, Agus M, Runfola M, Rebecchi F, Podda C, Moroni R. Analysis of the learning process for laparoscopic sleeve gastrectomy: CUSUM-curve of 110 consecutive patients with 1-year follow-up. J Visc Surg. 2021;158(3):198-203.
37.Ballesta-Lopez C, Poves I, Cabrera M, Almeida JA, Macías G. Learning curve for laparoscopic Roux-en-Y gastric bypass with totally hand-sewn anastomosis: analysis of first 600 consecutive patients. Surg Endosc. 2005;19(4):519-24
38.Sánchez-Santos R, Estévez S, Tomé C, González S, Brox A, Nicolás R, et al. Training programs influence in the learning curve of laparoscopic gastric bypass for morbid obesity: a systematic review. Obes Surg. 2012;22(1):34-41.
39.Bona D, Micheletto G, Bonitta G, Panizzo V, Cavalli M, Rausa E, et al. Does C-reactive Protein Have a Predictive Role in the Early Diagnosis of Postoperative Complications After Bariatric Surgery? Systematic Review and Bayesian Meta-analysis. Obes Surg. 2019;29(11):3448-3456.
40.Søvik TT, Aasheim ET, Kristinsson J, Schou CF, Diep LM, Nesbakken A, et al. Establishing laparoscopic Roux-en-Y gastric bypass: peri-operative outcome and characteristics of the learning curve. Obes Surg. 2009;19(2):158-165.
Full Text Sources:
Abstract:
Views: 531

For Authors
Journal Subscriptions

Mar 2024
Supplements
Instructions for authors
Online submission
Contact
e-ISSN: 2601 - 1700 (online)
ISSN-L: 2559 - 723X
Journal Abbreviation: Surg. Gastroenterol. Oncol.
Surgery, Gastroenterology and Oncology (SGO) is indexed in:
- SCOPUS
- EBSCO
- DOI/Crossref
- Google Scholar
- SCImago
- Harvard Library
- Open Academic Journals Index (OAJI)
Surgery, Gastroenterology and Oncology (SGO) is an open-access, peer-reviewed online journal published by Celsius Publishing House. The journal allows readers to read, download, copy, distribute, print, search, or link to the full text of its articles.
Time to first editorial decision: 25 days
Rejection rate: 61%
CiteScore: 0.2

Meetings and Courses in 2023
Meetings and Courses in 2022
Meetings and Courses in 2021
Meetings and Courses in 2020
Meetings and Courses in 2019
Verona expert meeting 2019

Surgery, Gastroenterology and Oncology applies the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits readers to copy and redistribute the material in any medium or format, remix, adapt, build upon the published works non-commercially, and license the derivative works on different terms, provided the original material is properly cited and the use is non-commercial. Please see: https://creativecommons.org/licenses/by-nc/4.0/
 IASGO Society News
IASGO Society News