Surgery, Gastroenterology and Oncology
|
|
Abstract
Introduction: Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the digestive system, with diverse histopathological and clinical features. This study aimed to analyze the clinical and pathological characteristics as well as surgical outcomes of GIST patients treated at our institution.
Methods: We retrospectively reviewed 49 patients who underwent surgery for GIST at Dicle University between 2010 and 2020. The collected data included demographic information together with tumor location and size measurements and mitotic rate counts and Fletcher risk classification and immunohistochemical marker results and surgical treatment details.
Results: The researchers evaluated mitosis among patients, 57.1% of whom were male, with a mean age of 58.8 ± 12.3 years. Tumors were primarily located in the small intestine (36%) and stomach (34%). Tumor sizes were <5 cm in 38.8%, 5-10 cm in 24.5%, and ³10 cm in 36.7%. Mitosis counts were £5 in 77.6% and >10 in 22.4%. Risk classification showed 52% low risk, 26% intermediate risk, and 22% high risk. All tumors were positive for CD34 and CD117. Ki-67 index was below 5% in 71.4% of cases. Postoperative mortality was 4%, and tumor recurrence occurred in 4.1%.
Conclusions: Effective surgical treatment combined with precise risk assessment is vital for managing GISTs. The study results confirm that adjuvant imatinib treatment reduces recurrence rates in patients with high-risk disease.
INTRODUCTION
Gastrointestinal stromal tumours (GIST) are the most common mesenchymal tumours of the gastrointestinal tract and account for approximately 0.1-3% of all gastrointestinal malignancies (1). These tumours arise from the precursors of the interstitial cells of Cajal, which regulate the peristaltic movements of the digestive tract and are known as the "pacemakers of the digestive system".
The global incidence ranges between 10-15 cases per million per year according to population-based studies (2). Although GISTs are most commonly seen in the stomach (55-60%), they can also occur in the small intestine (30-32%), colorectal region (6-7%), oesophagus (2%) and rarely in extraintestinal localisations such as peritoneum, mesentery, omentum and pancreas (3). The mean age at diagnosis is 60-70 years and there is no significant difference between genders (4).
Immunohistochemically, the majority of GISTs show CD117 (80-90%) and CD34 (60-70%) positivity and this feature is of critical importance in diagnosis (5). Mutations especially in KIT (CD117) and platelet-derived growth factor receptor alpha (PDGFRA) genes play an important role in GIST pathogenesis. SDH deficiency stands as a significant molecular alteration which contributes to GIST development in addition to KIT and PDGFRA mutations. SDH-deficient GISTs make up 5-7% of all GIST cases and they occur most frequently in patients with Carney triad who have gastric GIST and pulmonary chondroma and paraganglioma. The tumors primarily affect young patients while showing a preference for gastric sites and they respond differently to imatinib treatment (6,7).
The mutations have resulted in the development of targeted therapies such as tyrosine kinase inhibitors imatinib and sunitinib. The prognosis of the tumour largely depends on its size, mitotic number and location, and especially large tumours and those with a high mitotic index may have a more aggressive prognosis (8).
Although the epidemiology, clinical features and treatment approaches of GISTs are increasingly better understood in the literature, comprehensive studies revealing regional differences and reflecting the experiences especially in Turkey are limited (9). Although significant advances have been made in treatment with the introduction of tyrosine kinase inhibitors (imatinib), standardised approaches have not yet been clarified, especially regarding the follow-up protocols of high-risk patients and the management of patients with recurrence (1). Furthermore, there is no consensus in the literature regarding the natural history and optimal surgical strategies for small GISTs (10).
In this study, we aimed to contribute to this gap in the literature by sharing our single centre experience and to evaluate the relationship between the clinical features and surgical outcomes of GISTs in different localisations.
METHOD
Study Population and Sample
This retrospective cohort study included 49 patients who underwent surgery for GIST between January 2010 and December 2020 in the Department of General Surgery, Dicle University Faculty of Medicine. Since the number of patients included all patients treated for GIST in our clinic within the specified date range, no sample size calculation was made. Inclusion criteria were determined as being over 18 years of age, having a histopathological diagnosis of GIST and having undergone surgical treatment in our clinic. Exclusion criteria were defined as missing clinical or pathological data, primary treatment in another centre and presence of second primary malignancy. In our study, tumour size was defined as the largest diameter in centimetres; mitotic index was defined as the number of mitotic figures counted in 50 large magnification fields; and tumour localisation was classified as stomach, small intestine, colon, pancreas and extra-intestinal. Risk groups were divided into low, intermediate and high risk categories using the criteria (tumour diameter and number of mitoses) defined by Fletcher et al. In the evaluation of the number of mitoses, two categories were created: tumours with 5 or less mitoses and tumours with more than 10 mitoses.
Operating Procedures
Data were collected by retrospective review of patient files, operative notes and pathology reports. Demographic characteristics (age, gender), clinical characteristics (presenting complaints, diagnostic methods), tumour characteristics (localisation, size, mitosis number, risk group), immunohistochemical characteristics (CD34, CD117, Ki-67, Vimentin, SMA/ Desmin) and surgical treatment results (surgical method, complications, follow-up period, recurrence) were recorded on standard data collection forms. Histopathological examinations were performed by experienced pathologists according to standard protocols. Immunohistochemical features of the tumour were evaluated using appropriate antibody kits and CD117, CD34 and Ki-67 examinations were routinely performed on all tumours. Ki-67 proliferation index was analysed in three groups: below 5%, between 5-10% and above 10%. The follow-up of the patients was performed every 3 months in the first year, every 6 months in the second year, and then annual follow-ups in the postoperative period. Physical examination, abdominopelvic computed tomography and endoscopic examinations were performed when necessary. To ensure the reliability of the data collection process, the data were double checked by two independent investigators.
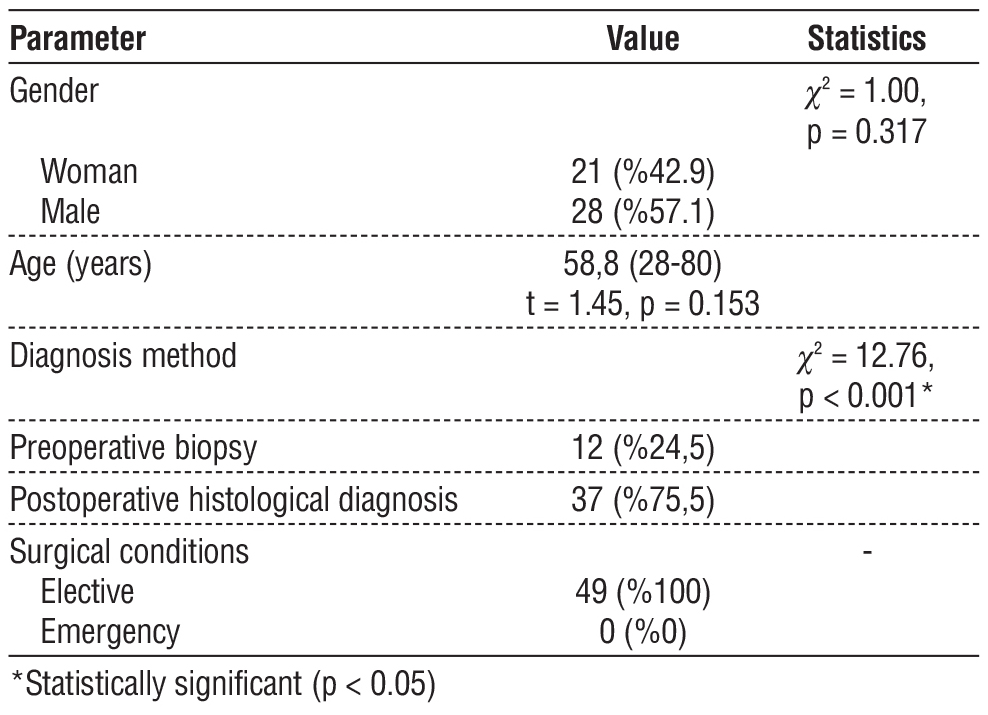
Table 1 - Demographic and clinical characteristics of the patients
Details of the Surgical Method
All patients underwent surgical treatment under elective conditions. The surgical method was determined according to the localisation, size and degree of invasion of the tumour. Wedge resection or subtotal gastrectomy was preferred for gastric tumours, segmentary resection and anastomosis for small bowel and colon tumours, Whipple procedure for pancreatic tumours and total mass excision for extraintestinal tumours. In all surgical procedures, negative surgical margins were tried to be achieved and care was taken to avoid tumour rupture. In cases with liver metastasis, metastasectomy was performed in addition to the primary surgical procedure. In the postoperative period, patients in the high-risk group received imatinib treatment for 3 years in the oncology clinic. Surgical intervention was performed again in patients with recurrence. All surgical procedures were performed by the same surgical team experienced in gastrointestinal surgery.
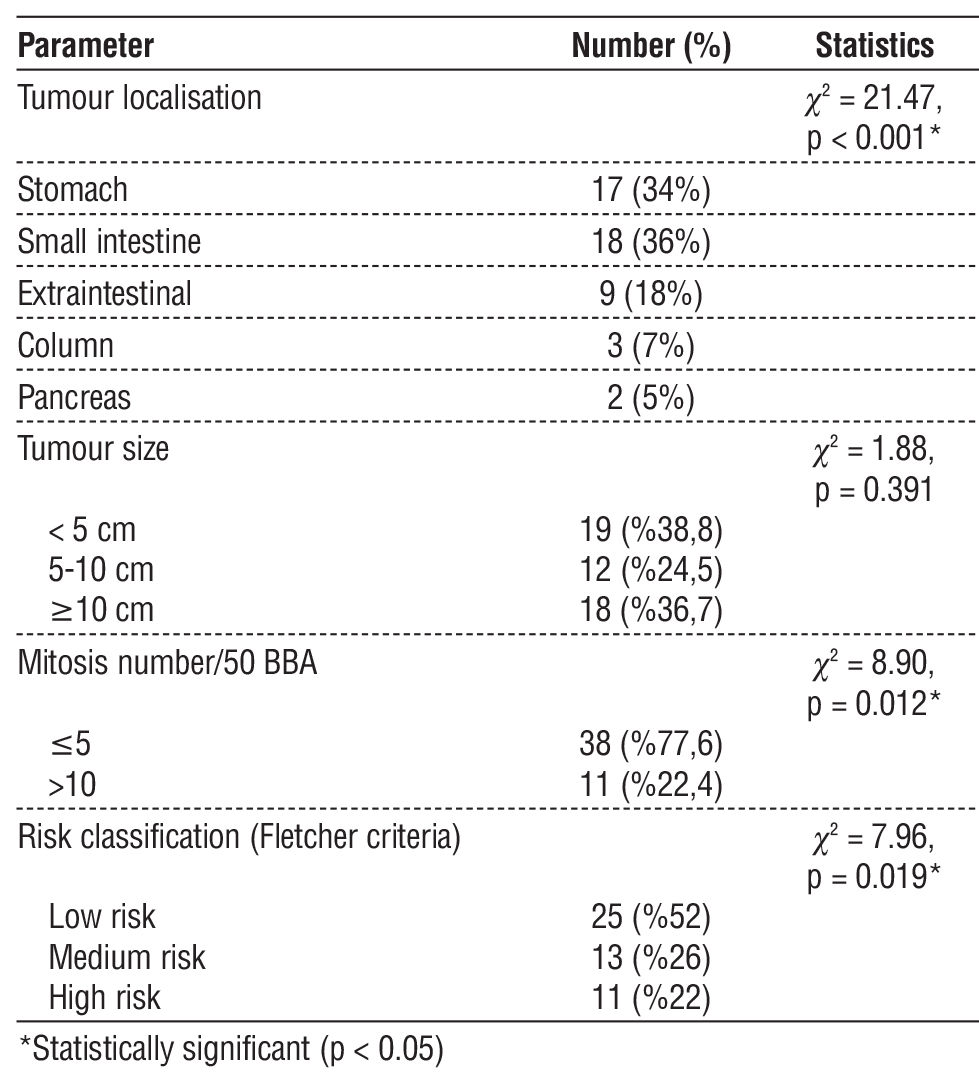
Table 2 - Tumour localisation and characteristics
Statistical Analysis
SPSS 22.0 (IBM Corp., Armonk, NY, USA) statistical software was used for data analysis. Descriptive statistics were presented as mean ± standard deviation (minimum-maximum) for continuous variables and as number and percentage for categorical variables.
Chi-square test or Fisher exact test was used in the comparison of categorical variables when the sample size was insufficient. In the comparison of continuous variables between independent groups, Student t-test or one-way analysis of variance (ANOVA) was used for normally distributed data, and Mann-Whitney U test or Kruskal-Wallis test was used for non-normally distributed data. Log-rank test was used to compare surgical treatment results and survival times. P value <0.05 was considered statistically significant. Since there were no missing data, no special method was used for missing data management. Subgroup analyses were performed according to tumour localisation and risk groups.
Ethical Considerations
This study was approved by the Dicle University Faculty of Medicine Non-Interventional Clinical Research Ethics Committee (Decision date: 09.04.2021, Number: 2021/253) or Internal Review Board (IRB). Patient data were analysed anonymised and the confidentiality of personal information was protected.
Human Ethics and Participation Consent Statements
Informed consent was obtained from all participants and the study was conducted in accordance with the Declaration of Helsinki.
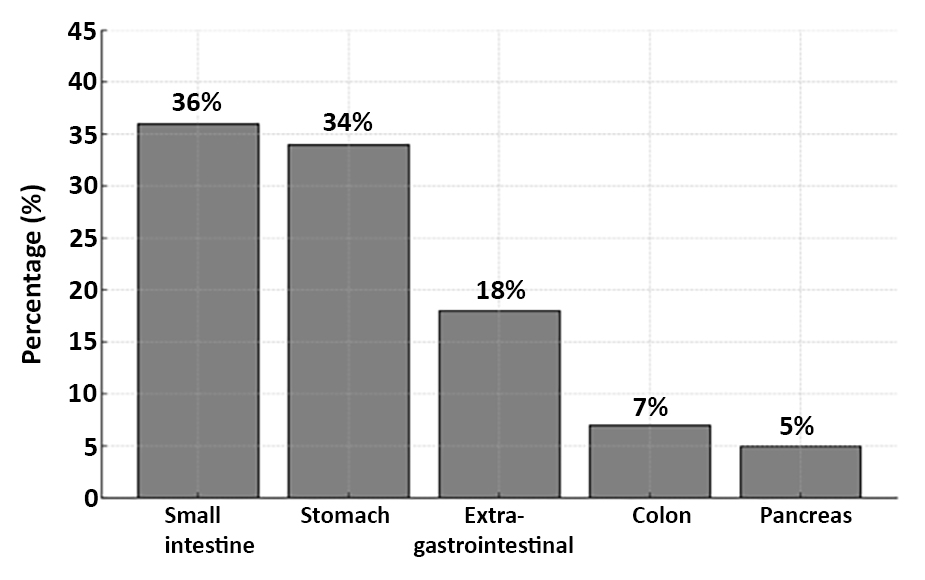
Figure 1 - Frequency Distribution of Tumor Localization in Gastrointestinal Stromal Tumors (GIST): This figure illustrates the relative frequency (%) of GIST tumor locations within the patient cohort (n=49), showing the small intestine as the most common site, followed by the stomach, extragastro-intestinal sites, colon, and pancreas. GIST refers to Gastrointestinal Stromal Tumors.
Informed Consent
For this retrospective study, individual informed consent was obtained from all patients as approved by the institutional review board.
RESULTS
Of the 49 GIST patients included in our study, 21 were female (42.9%) and 28 were male (57.1%). The mean age of the patients was 58.8±12.3 years and the age range was 28-80 years. When the diagnostic methods were examined, postoperative histological diagnosis was made in the majority of the patients (75.5%), while preoperative biopsy was performed in only 12 patients (24.5%). This difference was statistically significant (c2 = 12.76, p < 0.001). All of our patients were operated under elective conditions, and no condition requiring emergency surgical intervention was observed (table 1).
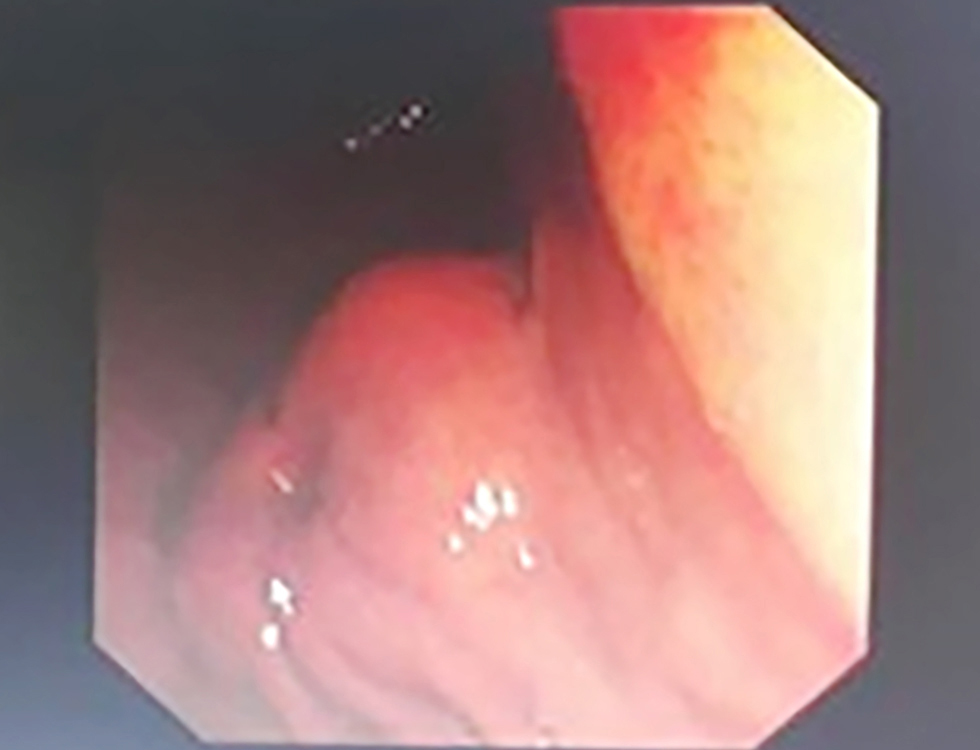
Figure 2 - Endoscopic view of the gastric antrum: Endoscopic image showing the gastric mucosa with potential submucosal lesion suspicious for GIST. (SCV: Scope Channel View; C:N Br:A1 G:O refers to endoscopic coordinates and settings with C:N indicating Color/Normal mode, Br:A1 indicating Brightness level A1, and G:O indicating Gain/Offset settings)
When the localisation of the tumours was evaluated, the most common localisation was small intestine (36%). This was followed by stomach (34%), extraintestinal localisation (18%), colon (7%) and pancreas (5%). This distribution was statistically significant (c2 = 21.47, p < 0.001). In terms of tumour size, the tumour diameter was less than 5 cm in 19 patients (38.8%), between 5-10 cm in 12 patients (24.5%), and 10 cm or larger in 18 patients (36.7%). There was no statistically significant difference between tumour size distributions (c2 = 1.88, p = 0.391). When the number of mitoses in 50 large magnification fields (MMS) were analysed microscopically, 38 (77.6%) patients had 5 or less mitoses and 11 (22.4%) patients had more than 10 mitoses. The difference between mitotic number distributions was statistically significant (c2 = 8.90, p = 0.012). According to the risk classification using Fletcher criteria, the majority of the patients (52%) were in the low risk group, 13 patients (26%) were in the intermediate risk group and 11 patients (22%) were in the high risk group (c2 = 7.96, p = 0.019) (table 2, fig. 1).
Table 3 - Immunohistochemical properties
Endoscopic examination revealed a submucosal, well-circumscribed mass lesion in the antrum of the stomach (fig. 2). This endoscopic appearance represents the typical presentation of our gastric GIST cases and similar endoscopic findings were observed in all gastric GIST cases.
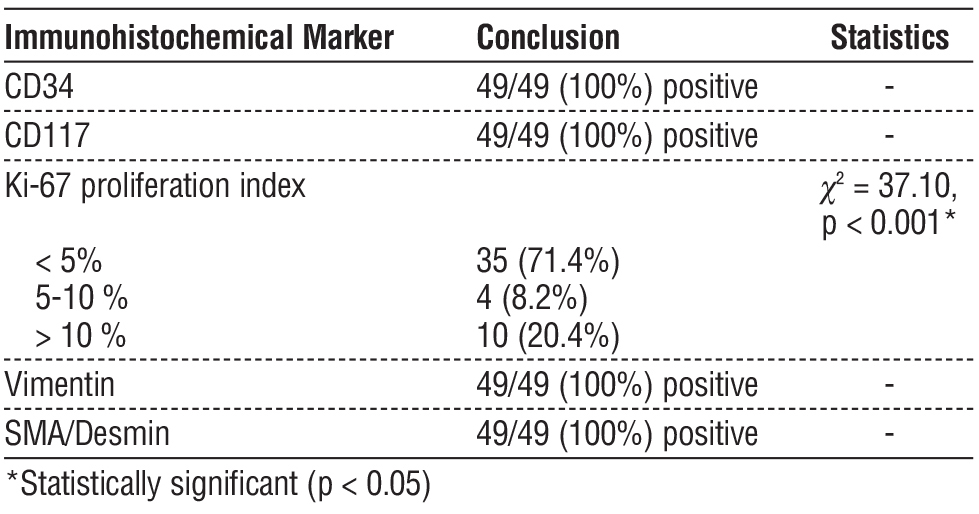
In immunohistochemical analyses, CD34, CD117, Vimentin and SMA/Desmin were positive in all tumours. When the Ki-67 proliferation index was analysed, it was found to be below 5% in the majority of patients (71.4%), between 5-10% in 8.2% and above 10% in 20.4%. These differences in Ki-67 index were statistically significant (c2 = 37.10, p < 0.001) (table 3).
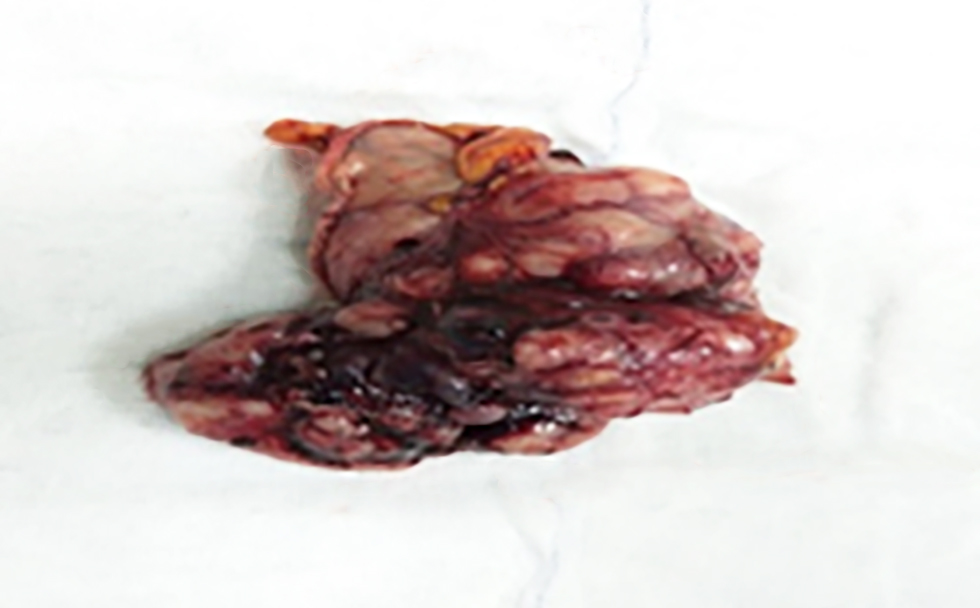
Macroscopic examination of the surgical specimen obtained after wedge resection for gastric GIST revealed a well-circumscribed, solid, submucosal mass with occasional haemorrhagic areas (fig. 3). This macroscopic appearance showed similar features in other GIST cases in our study.
Figure 3 - Surgical specimen after gastric wedge resection: Macroscopic view of the resected gastric GIST specimen showing a well-circumscribed, submucosal solid mass with areas of hemorrhage.
When the surgical treatments were analysed, 16 patients underwent small bowel resection+anastomosis and 3 patients underwent liver metastasectomy. In gastric tumours, 11 patients underwent wedge resection and 5 patients underwent subtotal gastrectomy. Total mass excision was performed in 9 patients with extraintestinal tumours, colon resection+anastomosis was performed in 3 patients with colon tumours, and Whipple procedure was performed in 2 patients with pancreatic tumours. When the follow-up periods were evaluated, the mean follow-up period for small bowel tumours was 17 months in patients who underwent liver metastasectomy and 21 months in patients who underwent bowel resection only. In gastric tumours, the mean follow-up time was 24 months in the wedge resection group and 18 months in the subtotal gastrectomy group. The follow-up period was 36 months in the extraintestinal group, 19 months in the colon group and 11 months in the pancreas group. There was no significant difference in survival between the surgical methods in patients who underwent small bowel surgery (Log rank = 2.31, p = 0.128) (table 4).
Table 4 - Surgical treatment and results
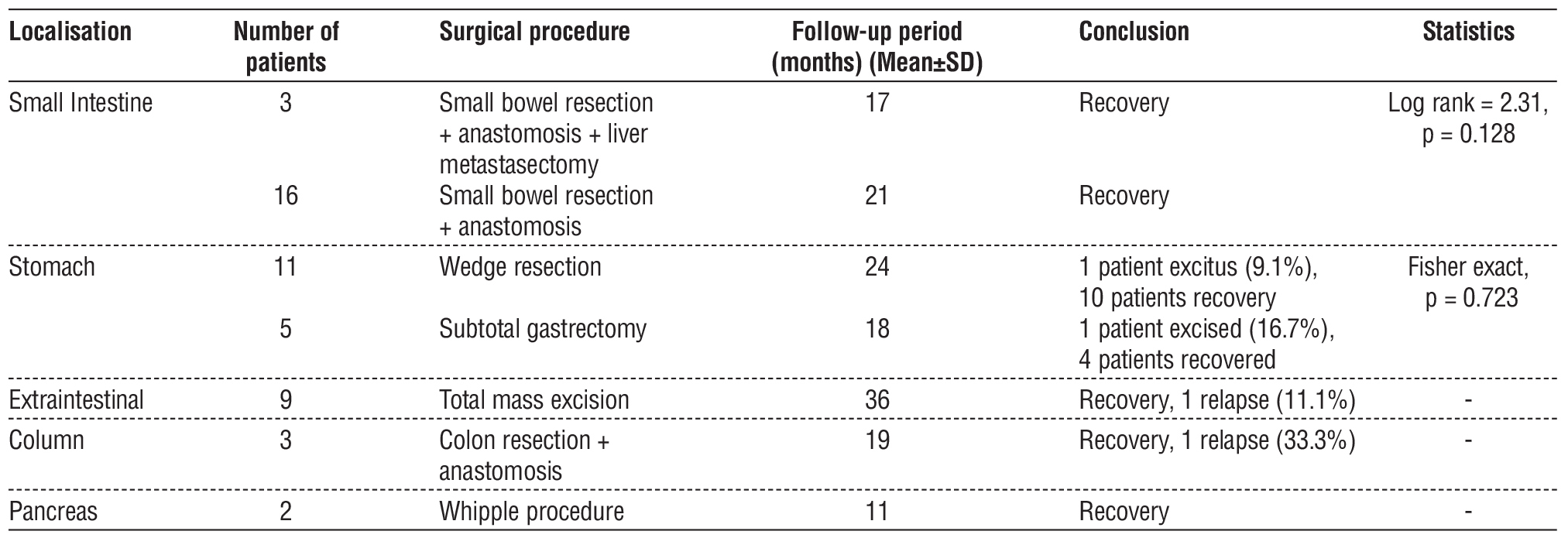
Table 5 - Complications and recurrence
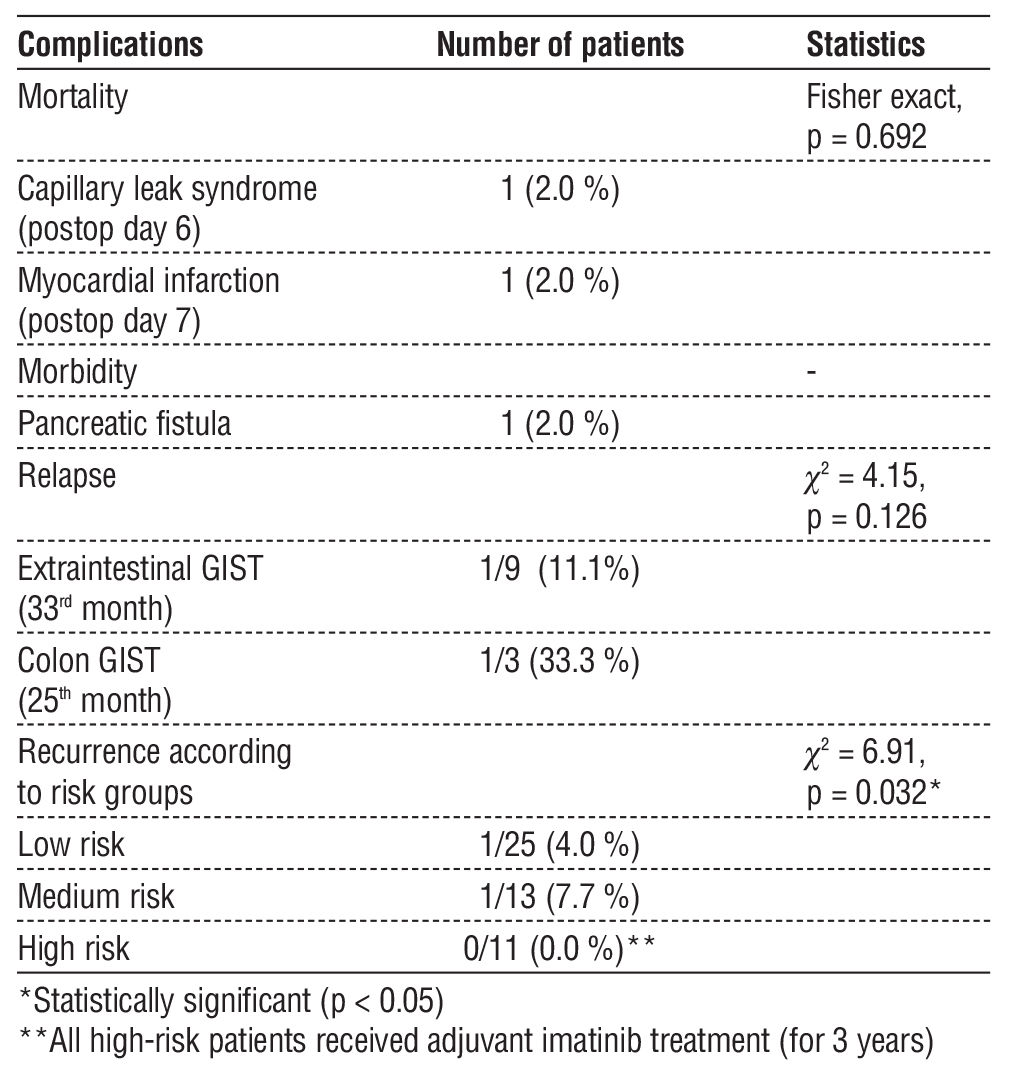
In terms of postoperative complications, mortality was observed in 2 patients (4.0%). One patient who underwent wedge resection died on postoperative day 6 due to capillary leak syndrome and the other patient who underwent subtotal gastrectomy died on post-operative day 7 due to myocardial infarction. Mortality rates were calculated as 9.1% and 16.7%, respectively, but there was no statistical difference between the two groups (Fisher exact, p = 0.723). As a morbidity, pancreatic fistula developed in one patient (2.0%) who underwent Whipple procedure, but closed spontaneously in the 2nd week of follow-up (table 5).
Recurrence was observed in two patients in long-term follow-up. One patient operated for low-risk extraintestinal GIST recurred at 33 months (11.1%) and one patient with intermediate-risk colonic GIST recurred at 25 months (33.3%). There was no statistically significant difference between the recurrence rates according to tumour localisation (c2 = 4.15, p = 0.126). These two patients were re-operated and no complications developed. When recurrence rates were compared according to risk groups, recurrence rates were 4.0% in the low-risk group and 7.7% in the intermediate-risk group, while no recurrence was observed in the high-risk group. All high-risk patients had received adjuvant imatinib treatment for 3 years in the postoperative period. The difference in recurrence rate between the risk groups was statistically significant (c2 = 6.91, p = 0.032) (table 5).
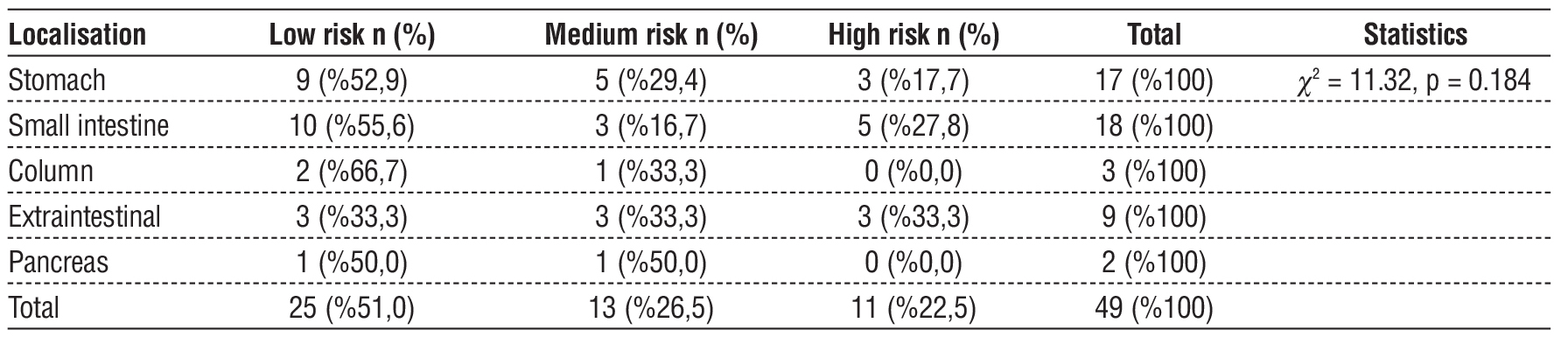
When the relationship between the localisation of tumours and risk groups was examined, 52.9% of gastric tumours were in the low risk group, 29.4% in the intermediate risk group and 17.7% in the high risk group. Among small intestinal tumours, 55.6% were in the low risk group, 16.7% in the intermediate risk group and 27.8% in the high risk group. In extraintestinal tumours, all three risk groups were equally distributed (33.3%). There were no high-risk cases among the tumours located in the colon and pancreas. In the colon, 66.7% of the cases were in the low risk group and 33.3% in the intermediate risk group, while 50.0% of the pancreatic tumours were in the low risk group and 50.0% in the intermediate risk group. However, this relationship between localisation and risk groups was not statistically significant (c2 = 11.32, p = 0.184) (table 6).
Table 6 - Relationship between tumour localisation and risk groups
DISCUSSION
The research evaluated both clinical aspects and pathological features of gastrointestinal stromal tumours together with surgical treatment results. Our research examined the distribution patterns and risk classification and long-term results of GIST patients treated at our center based on their tumor locations. The clinical progression and treatment responses of GISTs exhibit substantial variations according to our research findings. The management of rare mesenchymal tumours can be improved through understanding the relationships between tumour location and risk groups and surgical outcomes.
The majority of GIST cases in our study occurred in the small intestine at 36% and stomach at 34%. The study results showed different results than the gastric predominance which many studies in the literature have reported. Refai (2024) in Saudi Arabia reported that 66% of GIST cases were localised in the stomach (11). Similarly, a large population-based study using the SEER database reported that 63% of GISTs were localised in the stomach (12).
In terms of gender distribution, the rate of male patients in our study (57.1%) was found to be slightly higher than the rate of male patients in the SEER database study (52%), but lower than the male rates reported in Saudi Arabian and European studies (72% and 71.1%) (11,13). The mean age of 58.8±12.3 years in our study is largely compatible with the mean/median values of 60.5 years, 61.8 years and 62-64 years reported in studies in the literature (11,13,14). Regional differences, genetic factors and referral of cases with rare localisations due to the fact that our centre is a reference centre may explain the difference in the distribution of tumour localisation.
In our study, the tumour diameter was found to be less than 5 cm in 38.8% of the patients and 10 cm or larger in 36.7%. This distribution differs from the data reported in the study of Hashem et al. (2021); in their series, tumour size was ? 5 cm in only 14.7% of patients, while it was reported as >10 cm in 35.3% (15). In the study of Xu et al. (2021), the tumour size was reported to be <5 cm in 43.7% of the patients, which is closer to our results (16).
In the risk classification, the majority of our patients (52%) were in the low risk group, while 44.04% of the patients were classified in the low malignancy potential (very low and low risk) category in the study by Tian and Chen (2024) (17). In the study of Hashem et al. (2021), 33.3% of the patients were found in the high risk group according to the AFIP scheme, which is higher than the 22% rate in our study (15). The detection of Ki-67 proliferation index below 5% in 71.4% of our patients is compatible with low malignancy potential as stated by Tian and Chen (2024) (17).
In our study, organ-sparing surgical approaches as wedge resection (68.8%) and subtotal gastrectomy (31.3%) were preferred in gastric GISTs. This approach is consistent with the current approach reported by Cananzi et al. (2022), who considered R0 resection as the gold standard in surgical treatment (18). In our series, postoperative mortality rate was 4%, which is higher than the mortality rate (0%) observed in patients who underwent surgery after neoadjuvant imatinib treatment by Vassos et al. (2021) (19). In terms of morbidity, only one patient (2%) developed pancreatic fistula in our study, and similarly, pancreatic fistula was reported in one patient in the duodenal GIST study of Vassos et al. (2021) (20). The low complication rate in our series indicates the success of the surgical technique.
The immunohistochemical analysis included CD117 and CD34 and Ki-67 markers but researchers should evaluate additional prognostic indicators. The loss of SDHB immunostaining identifies SDH-deficient GISTs as a specific molecular group which shows different clinical characteristics and treatment options. The authors of Constantin et al. (2014) stressed that GIST diagnosis requires team-based care for emergency surgical cases (6). The study by Ceausu et al. (2021) showed how these tumors create difficulties for diagnosis. Researchers should add SDHB immunostaining to standard diagnostic tests because it helps identify patients who are young and have gastric tumors or show symptoms of syndromes (7).
In patients with advanced GIST, Li et al. (2024) reported that there was no significant difference in overall survival between patients with and without surgery (76.5 months vs. 78.9 months) (21). This finding suggests that the role of surgery may be limited in metastatic patients. In our series, liver metastasectomy was performed in 15.8% (3/19) of small bowel tumours, indicating the aggressiveness of the surgical approach.
The low recurrence rate of 4.1% found in our study in long-term follow-up is compatible with some studies in the literature. Jakob et al. (2022) reported similarly low recurrence rates in their study of 350 patients, and local recurrence was observed in only one patient (22).
Interestingly, it is noteworthy that the patients with recurrence in our study were in the low and intermediate risk groups, while no recurrence was observed in the high risk group. This unexpected finding is supported by the statistically significant difference in the recurrence rate between the risk groups (p=0.032) and may be explained by the efficacy of adjuvant imatinib treatment in high-risk patients, as shown in the study by Blay et al. In our series, adjuvant imatinib treatment was administered to all high-risk patients for 3 years, and the efficacy of longer-term treatment is also reported in the literature. Blay et al. showed that 6 years of imatinib treatment reduced the recurrence rate up to 28% in high-risk localised GIST patients (23).
In addition, the fact that recurrence developed in a case of extragastrointestinal GIST in our study supports the finding reported by Feng et al. (2021) that extragastrointestinal stromal tumours may have worse disease-free survival (24). When the relationship between tumour localisation and risk groups is evaluated, the fact that stomach and small intestine tumours are mostly in the low-risk group, although not mentioned in the study by Stavrou et al. (2025), is in line with the general observations in the literature (25). The fact that there were no high-risk cases in tumours located in the colon and pancreas may be due to the limited sample size.
Our study has some limitations. The study results lack general applicability because it used retrospective design and data from a single center. The small number of patients in the study reduced statistical power particularly for GISTs that occur infrequently in different locations. The study benefits from its extended follow-up duration and thorough immunohistochemical assessments and complete risk group classification system. Future research should investigate how molecular markers affect patient outcomes while comparing surgical methods and assessing the success of additional treatments. In addition, the establishment of a multicentre GIST database in our country will strengthen evidence-based approaches in the management of these rare tumours.
CONCLUSION
In conclusion, GISTs are the most common mesenchymal tumours of the gastrointestinal tract and their clinical course and treatment responses are variable. In our study, it was found that the majority of GIST cases were located in the small intestine and stomach and more than half of the patients were in the low risk group. The surgical treatment with organ-preserving methods achieved low rates of complications and recurrence. The follow-up and treatment planning of patients received guidance from immunohistochemical markers and risk classification systems.
A multidisciplinary approach with suitable surgical strategy enables successful treatment of GIST patients. The long-term reduction of recurrence rates in high-risk patients can be achieved through adjuvant imatinib treatment.
Conflict of Interest
The authors declare that they have no conflict of interest to disclose.
Funding
This study did not receive financial support.
Data Availability
Data used in this study can be provided on reasonable request.
REFERENCES
1. El-Menyar A, Mekkodathil A, Al-Thani H. Diagnosis and management of gastrointestinal stromal tumors: An up-to-date literature review. J Cancer Res Ther. 2017;13:889-900.
2. Søreide K, Sandvik OM, Søreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. 2016;40:39-46.
3. Sanchez-Hidalgo JM, Duran-Martinez M, Molero-Payan R, Rufian-Peña S, Arjona-Sanchez A, Casado-Adam A, et al. Gastrointestinal stromal tumors: A multidisciplinary challenge. World J Gastro-enterol. 2018;24(18):1925-1941.
4. Parab TM, DeRogatis MJ, Boaz AM, Grasso SA, Issack PS, Duarte DA, et al. Gastrointestinal stromal tumors: a comprehensive review. J Gastrointest Oncol. 2019;10(1):144-154.
5. Wu CE, Tzen CY, Wang SY, Yeh CN. Clinical diagnosis of gastro-intestinal stromal tumor (GIST): from the molecular genetic point of view. Cancers (Basel). 2019;11(5):679.
6. Constantin VD, Socea B, Popa F, Carâp AC, Gheorghe Popescu, Teodora Vlãdescu, et al. A histopathological and immunohisto-chemical approach of surgical emergencies of GIST. An interdisciplinary study. Rom J Morphol Embryol. 2014;55(2 Suppl):619-627.
7. Ceausu M, Socea B, Ciobotaru VP, Constantin VD, Enache S, Enache V, et al. A multidisciplinary approach in the diagnostic challenge of GIST. Exp Ther Med. 2021;22(4):1063.
8. Belfiori G, Sartelli M, Cardinali L, Tranà C, Bracci R, Gesuita R, et al. Risk stratification systems for surgically treated localized primary gastrointestinal stromal tumors (GIST). Review of literature and comparison of the three prognostic criteria: MSKCC Nomogram, NIH-Fletcher and AFIP-Miettinen. Ann Ital Chir. 2015;86(3):219-27.
9. McDonnell MJ, Punnoose S, Viswanath YKS, Wadd NJ, Dhar A. Gastrointestinal stromal tumours (GISTs): an insight into clinical practice with review of literature. Frontline Gastroenterol. 2017; 8(1):19-25.
10. Nishida T, Goto O, Raut CP, Yahagi N. Diagnostic and treatment strategy for small gastrointestinal stromal tumors. Cancer. 2016; 122(20):3110-8.
11. Refai F. Gastrointestinal stromal tumors: a retrospective study at a tertiary care center in Saudi Arabia in the last decade. Cureus. 2024;16(7):e64560.
12. Khan J, Ullah A, Waheed A, Karki NR, Adhikari N, Vemavarapu L, et al. Gastrointestinal stromal tumors (GIST): a population-based study using the SEER database, including management and recent advances in targeted therapy. Cancers. 2022;14(15):3689.
13. Mohammadi M, IJzerman NS, Hohenberger P, Rutkowski P, Jones RL, Martin-Broto J, et al. Clinicopathological features and treatment outcome of oesophageal gastrointestinal stromal tumour (GIST): a large, retrospective multicenter European study. Eur J Surg Oncol. 2021;47(8):2173-81.
14. Sharma AK, de la Torre J, IJzerman NS, Sutton TL, Zhao B, Khan TM, et al. Location of gastrointestinal stromal tumor (GIST) in the stomach predicts tumor mutation profile and drug sensitivity. Clin Cancer Res. 2021;27(19):5334-42.
15. Hashem WB, El-Nahas TH, Fawzy M, Mashhour SN, Zedan MH, Mashhour KN. Gastrointestinal stromal tumor: clinicopathological features, management, and comparison of three risk stratification schemes. Res Oncol. 2021;17(2):73-79.
16. Xu SJ, Zhang SY, Dong LY, Lin GS, Zhou YJ. Dynamic survival analysis of gastrointestinal stromal tumors (GISTs): a 10-year follow-up based on conditional survival. BMC Cancer. 2021;21:1170.
17. Tian J, Chen W. Prediction of Ki-67 expression and malignant potential in gastrointestinal stromal tumors: novel models based on CE-CT and serological indicators. BMC Cancer. 2024;24:1412.
18. Cananzi FCM, Ruspi L, Samà L, Renne SL, Sicoli F, Quagliuolo V. The gist of surgical margins in GIST: a narrative review. Laparosc Surg. 2022;6:4.
19. Vassos N, Jakob J, Kähler G, Reichardt P, Marx A, Dimitrakopoulou-Strauss A, Rathmann N, Wardelmann E, Hohenberger P. Preservation of organ function in locally advanced non-metastatic gastrointestinal stromal tumors (GIST) of the stomach by neoadjuvant imatinib therapy. Cancers. 2021;13(4):586.
20. Vassos N, Perrakis A, Hohenberger W, Croner RS. Surgical approaches and oncological outcomes in the management of duodenal gastrointestinal stromal tumors (GIST). J Clin Med. 2021;10(19):4459.
21. Li J, Huang Z, Zhou H, Li H, Zhang J. Survival outcomes and prognostic factors of advanced gastrointestinal stromal tumors: in the era of multiple tyrosine kinase inhibitors. J Gastrointest Oncol. 2024;15(3):931-945.
22. Jakob J, Salameh R, Wichmann D, Charalambous N, Zygmunt AC, Kreisel I, et al. Needle tract seeding and abdominal recurrence following pre-treatment biopsy of gastrointestinal stromal tumors (GIST): results of a systematic review. BMC Surg. 2022;22:202.
23. Blay JY, Schiffler C, Bouché O, Brahmi M, Duffaud F, Toulmonde M, et al. A randomized study of 6 versus 3 years of adjuvant imatinib in patients with localized GIST at high risk of relapse. Ann Oncol. 2024;35(12):1157-66.
24. Feng H, Hu W, Zheng C, Wang W, Zheng G, Feng X, et al. Clinical features of extragastrointestinal stromal tumor compared with gastrointestinal stromal tumor: a retrospective, multicenter, real-world study. J Oncol. 2021;2021:1460131.
25. Stavrou N, Memos N, Filippatos C, Sergentanis TN, Zagouri F, Gavriatopoulou M, et al. Neoadjuvant imatinib in recurrent/metastatic gastrointestinal stromal tumors: a systematic review and meta-analysis of proportions. J Gastrointest Cancer. 2025;56:88.
Full Text Sources:
Abstract:
Views: 20

For Authors
Journal Subscriptions

Sept 2025
Supplements
Instructions for authors
Online submission
Contact
e-ISSN: 2601 - 1700 (online)
ISSN-L: 2559 - 723X
Journal Abbreviation: Surg. Gastroenterol. Oncol.
Surgery, Gastroenterology and Oncology (SGO) is indexed in:
- SCOPUS
- EBSCO
- DOI/Crossref
- Google Scholar
- SCImago
- Harvard Library
- Open Academic Journals Index (OAJI)
Surgery, Gastroenterology and Oncology (SGO) is an open-access, peer-reviewed online journal published by Celsius Publishing House. The journal allows readers to read, download, copy, distribute, print, search, or link to the full text of its articles.
Time to first editorial decision: 25 days
Rejection rate: 61%
CiteScore: 0.2

Meetings and Courses in 2025
Meetings and Courses in 2024
Meetings and Courses in 2023
Meetings and Courses in 2022
Meetings and Courses in 2021
Meetings and Courses in 2020
Meetings and Courses in 2019
Verona expert meeting 2019

Surgery, Gastroenterology and Oncology applies the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits readers to copy and redistribute the material in any medium or format, remix, adapt, build upon the published works non-commercially, and license the derivative works on different terms, provided the original material is properly cited and the use is non-commercial. Please see: https://creativecommons.org/licenses/by-nc/4.0/
Publisher’s Note:
The opinions, statements, and data contained in article are solely those of the authors and not of Surgery, Gastroenterology and Oncology journal or the editors. Publisher and the editors disclaim responsibility for any damage resulting from any ideas, instructions, methods, or products referred to in the content.
 IASGO Society News
IASGO Society News