Surgery, Gastroenterology and Oncology
|
|
Abstract
Background: Dieulafoy’s lesion, an uncommon cause of upper nonvariceal bleeding, is a submucosal vessel that erodes the near normal appearing mucosa without primary ulceration or erosion. The lesion occurs predominantly in the stomach, but it is also reported in extragastric sites.
Case and review of the literature: We present the case of a 69 years old patient, who presented in the emergency department with massive upper gastrointestinal bleeding caused by a duodenal Dieulafoy’s lesion. Risk factors included: chronic renal failure on hemodialysis and low-dose Aspirin intake. CARE (CAse REports) guidelines were followed, and CARE-writer online application was used. We also performed a systematic review of available data literature, following PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines. An extensive literature search was performed using the following keywords: Dieulafoy lesion, duodenal bulb, case report, series. We searched databases such as PubMed, Embase, Google Scholar and Cochrane, as well as references of studies of screened articles to extract data about relevant articles.
Results: The review outlines current understanding of the epidemiology, risk factors and clinical importance of Dieulafoy’s lesion as well as the currently available approaches to diagnosis and management.
Conclusions: We would also like to underline the importance of urgent endoscopy in diagnosis and treatment, delayed endoscopy being probably responsible for the ”iceberg effect” on the current available data.
BACKGROUND
Dieulafoy lesion has recently been described as ”the little known sleeping giant of gastrointestinal bleeds” (1), which we find perfectly matching our presented case.
The lesion was first described by Gallard in 1884 (2). Later on, Paul Georges Dieulafoy was the first pathologist and surgeon who described a series of patients who presented with fatal massive acute gastrointestinal bleeding, due to a minimal superficial erosion of a submucosal arteriole, described as ”exulceratio simplex” or later ”Dieulafoy lesion” (3,4). The essential typical characteristic is the fact that the nearby mucosa is in almost or perfect health, the lesion being quite different from a basic gastric or duodenal ulcer (5). First cases were described in the stomach (6-10), but later literature described localization almost everywhere along the gastrointestinal tract from the esophagus (10-13), duodenum and small bowel (14-24), colon and rectum (25,26), as well as the trachea (27) or bronchus (28-30). Besides ”exulceratio simplex”, other descriptions included: ”caliber persistent artery”, ”gastric aneurysm”, ”gastric arterio-sclerosis”, ”submucosal arteriolar malformation” and ”cirsoid aneurysm” (1,2,31).
Etiology remains unknown, but no causality has been established between Dieulafoy lesion and other risk factors such as use of non-steroidal anti-inflammatory drugs, alcohol, smoking, previous ulcer peptic disease or Helicobacter Pylori infection (10,32,33).
Dieulafoy lesion accounts for about 1% of all upper gastrointestinal bleedings, varying between 0.5-14% (3,34,35). However, it is possible that an ”iceberg effect” as a lack of correct diagnosis and underrecognizing is responsible, rather than a true rarity of the lesion (34,35). Intermittent bleeding on normal appearing mucosa covering the submucosal arteriole and especially delayed endoscopy could make it almost impossible to diagnose the lesion as it may not be visible once the bleeding has stopped for the moment (3,35).
REVIEW OF THE LITERATURE
CARE (CAse REports) guidelines were followed, and CARE-writer on-line application was used.
We also performed a systematic review of available data literature, following PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines.
An extensive literature search was performed using the following keywords: Dieulafoy lesion, duodenal bulb, case report, series. We searched databases such as PubMed, Embase, Google Scholar and Cochrane, as well as references of studies of screened articles to extract data about relevant articles.
CASE REPORT
A 69 years old woman presented to our emergency department for massive hematemesis and 3 days of black tarry stools, followed by generalized body weakness. Medical history included chronic renal failure, the patient is scheduled for hemodialysis 3 times per week. She is also known with chronic anemia (last hemoglobin value being 11g/dl, in the context of chronic renal failure), high blood pressure under treatment. Chronic medication also included Aspirin for maintaining the patency of the vascular fistula used for hemodialysis. She denied recent other non-steroidal anti-inflammatory drugs (NSAID) use.
At presentation, hemoglobin level was 7.1g/dl, the patient was hemodynamically unstable with blood pressure of 90/50 mmHg and a ventricular rate of 110 beats per minute. Pre-endoscopic Rockall score was 6 points. In the emergency department, the patient was stabilized. Proton pump inhibitors (PPI) were initiated according to current guidelines on upper gastrointestinal bleeding (36,37).
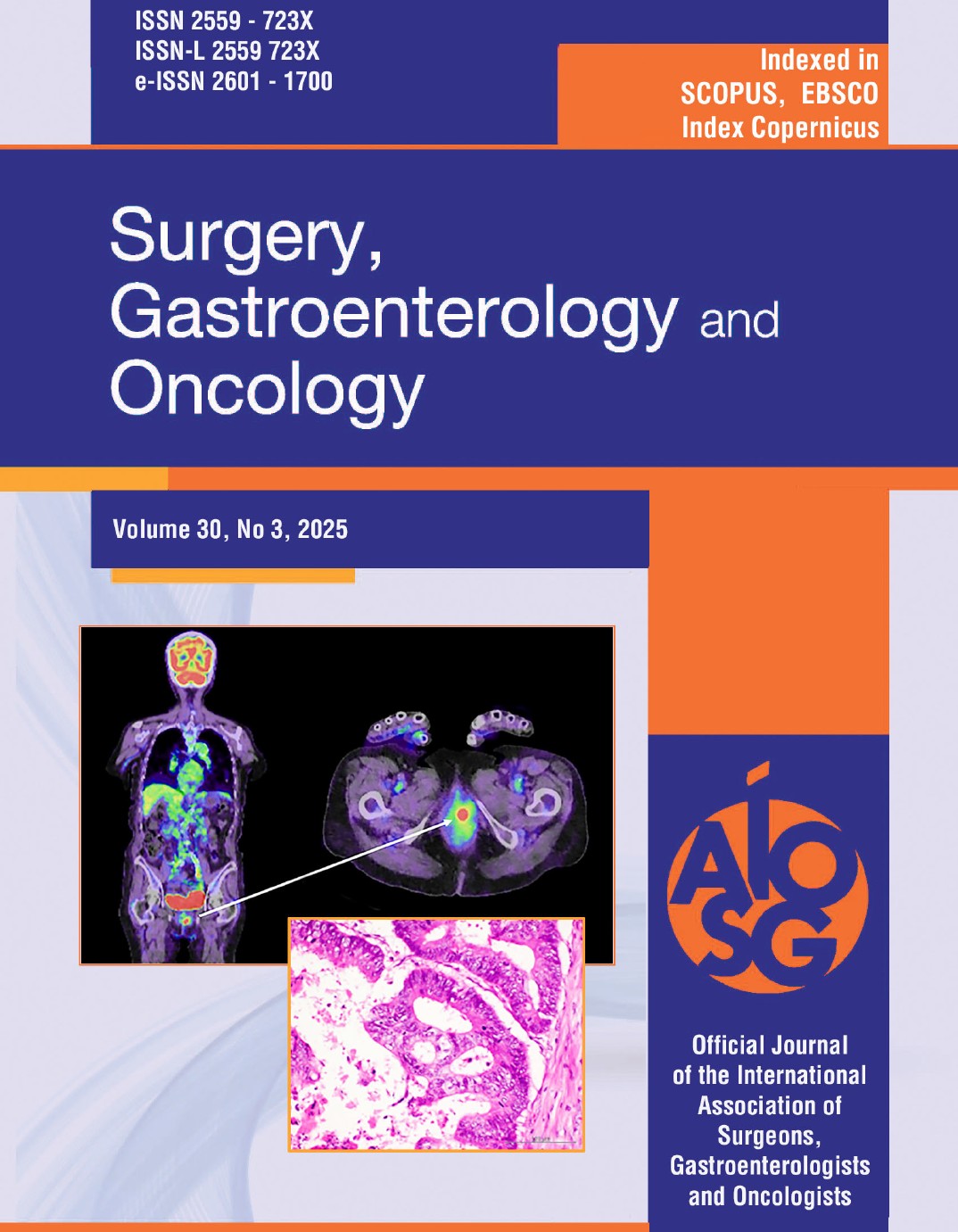
Due to episodes of hemodynamic instability and high-risk comorbidities (renal failure on hemodialysis), urgent upper endoscopy was performed, which revealed active massive upper gastrointestinal bleeding, being quite difficult to initially exactly identify the bleeding source. After intensive washing, we found a bulbar Dieulafoy lesion, with a practically normal surrounding surface mucosa but active continuous bleeding, as depicted in the images below. Endoscopic hemostasis was performed: first we injected 10 ml of diluted adrenalin solution 1:10.000, in an attempt to lower the bleeding rate and ”clean’’ the bleeding site, as well as to help us identify the exact lesion and the possible localization of the submucosal artery, in order to intuitively guide us when placing the hemoclips. Bleeding persisted as relatively massive oozing after injection therapy. The first clip placement attempt failed. Afterwards, 3 hemoclips were successfully placed and the bleeding eventually stopped (figs. 1-6).
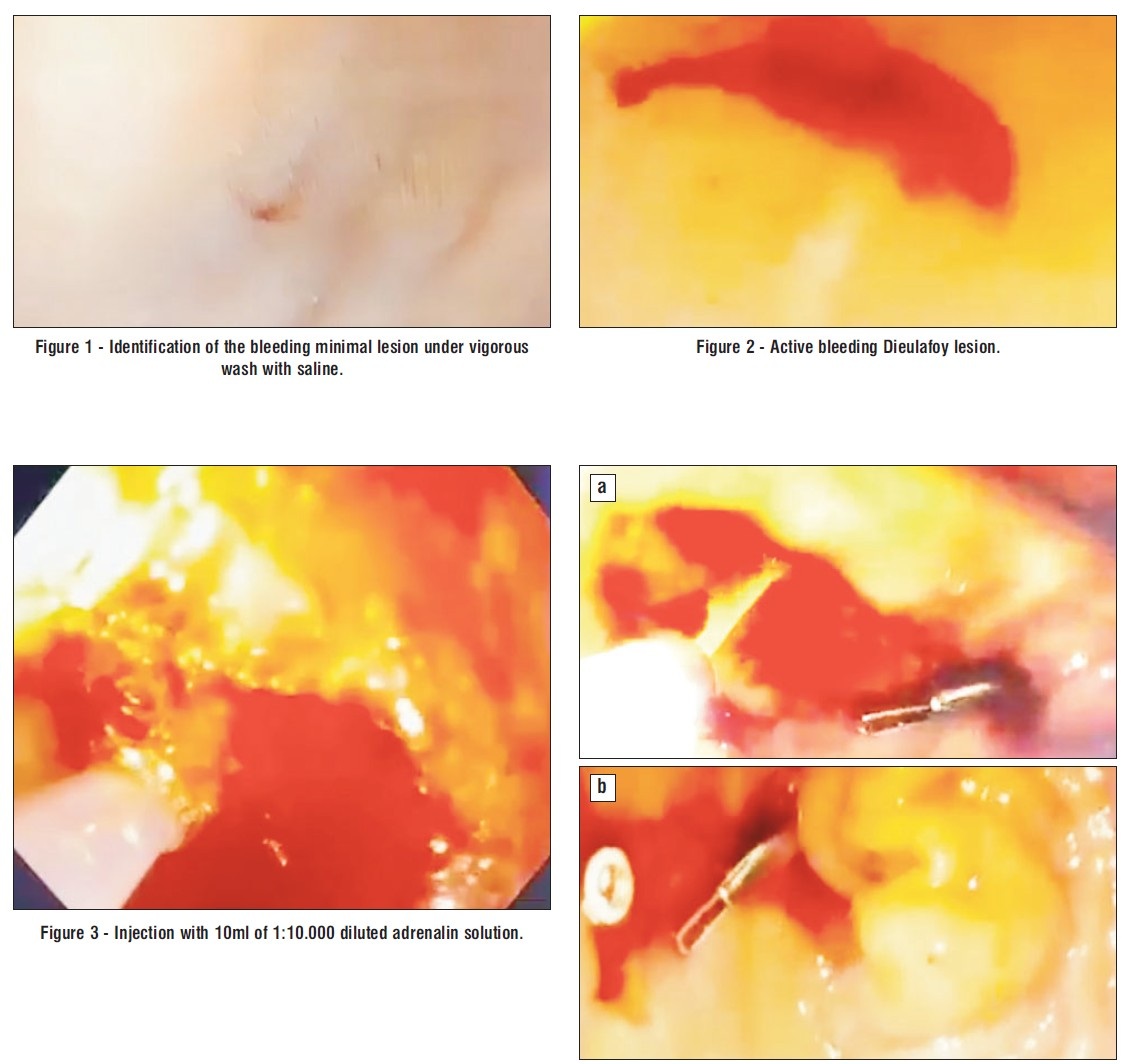
Figure 4 c - Placement of first hemoclip, one hemoclip failed before.

Figure 5 a,b,c - Placement of second hemoclip.
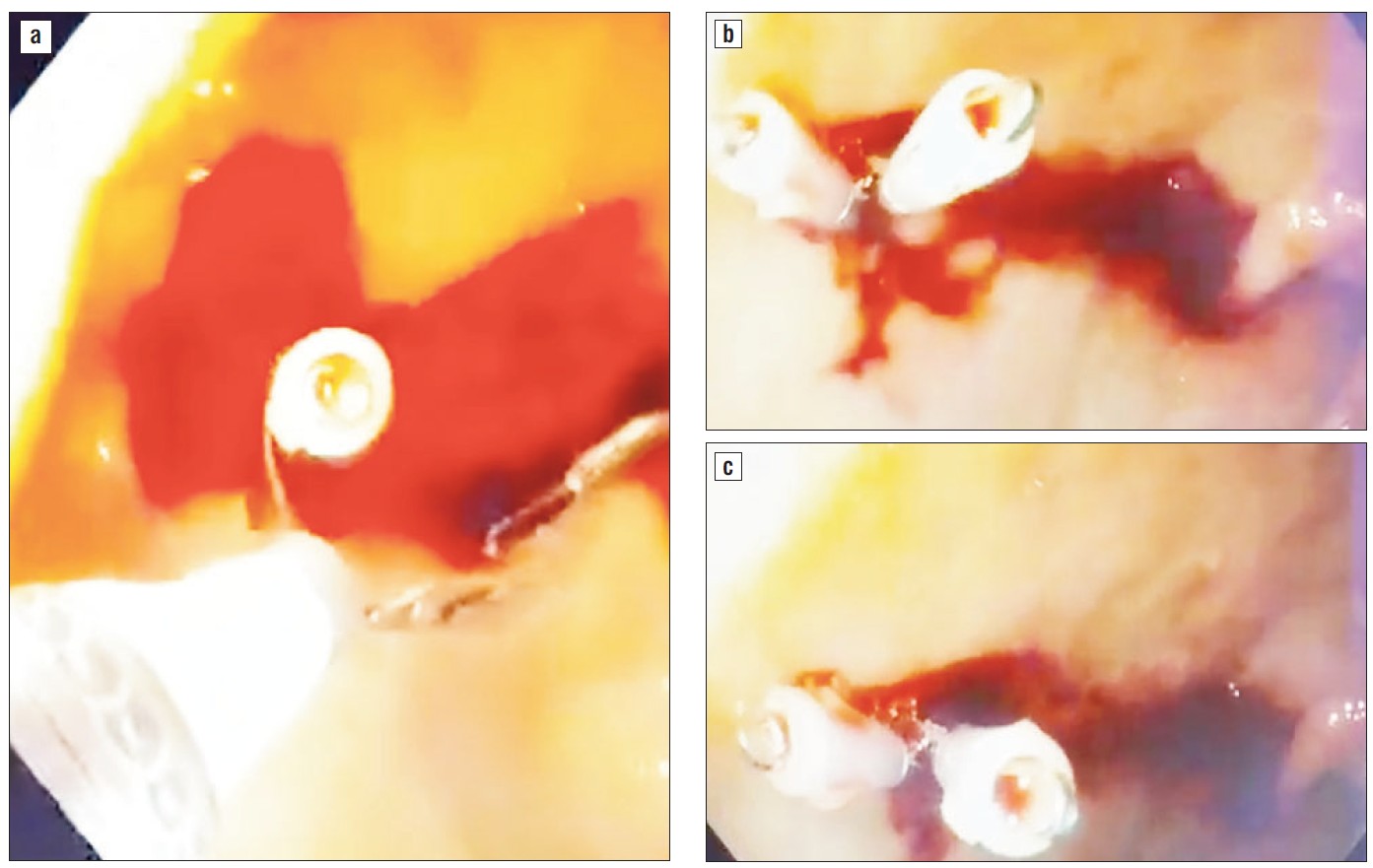

Figure 6 a,b - Placement of third hemoclip.
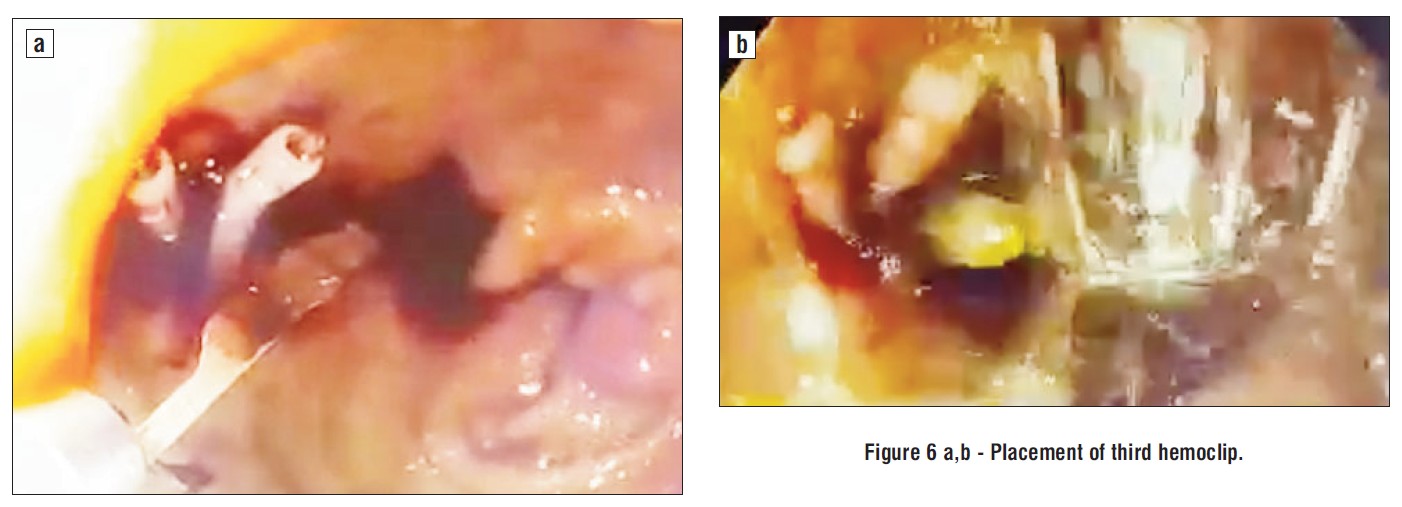
Following endoscopic treatment, Aspirin was stopped for the next 5 days, proton pump inhibitors were continuously administered at o rate of 8 mg/hour for the next 72 hours, and peritoneal dialysis was performed the second day after endoscopic hemostasis. Clinical evolution was favorable, the patient didn’t rebleed and no major gastrointestinal events were encountered the following weeks. At 3 months follow-up, the patient did not rebleed.
DISCUSSIONS
Localization
The most common encountered localization of gastrointestinal Dieulafoy lesion is within 6 cm of the gastro-esophageal junction, mostly on the lesser gastric curvature, possibly due to the particular anatomic vascularization of this region (3). A significant proportion of cases were found at intestinal anastomoses, predominantly Billroth II anastomosis after gastrectomy (29,31). Though multiple extra-gastric localization are possible, duodenal one was once considered to be rare, the first one being reported in 1988 (38).Almost ten years later, on a large series of 100 cases of Dieulafoy’s lesion, Veldhuyzen found no duodenal localization (9). However, possibly due to increased awareness of the disease and development of endoscopic diagnosis and hemostasis, several recent studies (10-26) have reported more extra-gastric localization cases: in the light of new data, the duodenal lesion has become the most frequently non-gastric lesion, followed by the colon (10%), jejunum (2%) and esophagus (2%) (31). Though extremely rare, even gall-bladder Dieulafoy lesion has been reported as a cause for haemobilia (39). Extra-digestive localization is rare but has also been reported in the literature in cases of tracheal or bronchial Dieulafoy’s lesion (27-30).
Diagnosis
Clinical presentation consists of painless recurrent intermittent hematemesis or melena/hematochezia and hemodynamic instability ranging from general weakness to syncope or hemorrhagic shock (7,8). Clinical presentation may depend on the duration of the bleeding, general condition and comorbidities of the patient, localization and size of the bleeding vessel (2,14,17).
In the past preendoscopic era, the diagnosis was rarely made before surgery or post-mortem examination (3,5,6). With increasing endoscopic experience, as well as increased successful endoscopic hemostasis, diagnosis is now based more on endoscopic criteria rather than histopathological ones (35,40,41).
The hemodynamic status is also important for deciding on diagnosis modalities, most of the patients are initially evaluated by upper endoscopy or colono-scopy, especially in non-tertiary gastro-enterological centers (32,42).
Even though index endoscopy may be effective for diagnosis for up to 70% of the patients, several endoscopies may be necessary, especially when bleeding has initially stopped (24,31). About 6% of the patients may require more than 3 endoscopies to establish the
correct diagnosis (6). Risk factors for missing the lesion include: excessive blood, clots and sometimes (as in our case) very subtle lesions on normal appearing mucosa, the intermittent nature of hemorrhages (24,31,34).
Several endoscopic criteria have been proposed for Dieulafoy lesions:
active or pulsing arterial bleeding from a minimal defect of the mucosa with normal surrounding mucosa;
endoscopic visualization of a vessel that protrudes, with or without active bleeding, through a minimal defect of the mucosa or through surrounding normal mucosa;
fresh clotting, adhering at a narrow point of attachment to a minimal defect of the mucosa or to mucosa of normal appearance (31).
When considering angiodysplasia (located in the small bowel and from an endoscopic point of view), some authors (43,44) have classified lesions into 6 groups. In this classification, Dieulafoy lesion may be considered a type 2a or b angiodysplasia. Angiodysplasias are classified into the following 6 groups: type 1a, punctulate erythema (<1 mm), with or without oozing; type 1b, patchy erythema (a few mm), with or without oozing; type 2a, punctulate lesions (< 1 mm), with pulsatile bleeding; type 2b, pulsatile red protrusion, without surrounding venous dilatation; type 3, pulsatile red protrusion, with surrounding venous dilatation; type 4, other lesions not classified into any of the above categories.
Several alternative diagnosis methods include enteroscopy, capsule endoscopy, angiography, red cell scanning and surgical exploration, especially when the other methods fail (31,36,37,45). Barium studies were unsuccessful in the diagnosis of duodenal Dieulafoy’s lesions (24).
Enteroscopy allows evaluation of the small intestine up to 150 cm from the pylorus, thus diagnosing 70-100% of patients with occult gastrointestinal bleeding. Push, single- or double-balloon enteroscopy can be used for both diagnosis and treatment (34). The decision as to whether the initial approach of enteroscopy is oral or anal is based on clinical presentation or the result of capsule endoscopy. Tattooing is sometimes used to mark the bleeding site but also to mark the deepest insertion point when the bleeding spot has not been localized. It was reported that at least 2 or more procedures may be necessary before diagnosis. Intraoperative enteroscopy from an enterotomy is also a diagnosis possibility, especially in distal lesions (31,34,46).
Capsule endoscopy is a valuable noninvasive tool but has the great disadvantage of not allowing endoscopic treatment. However, it is very useful in guiding therapeutic interventions in selected cases (47,48).
Angiography and red cell scanning can be used to pinpoint the location of jejunal/small intestine bleed (31,49). There is no specific radiological view of the lesion. Angiographic findings include extravasation of contrast from an eroded artery that may appear normal (50). If standard angiography fails, heparin may be used to help identify the bleeding site (31,52). However, existence of a tortuous and ectatic artery in angiography can provide important clues for the diagnosis (53). Technetium-99m labeled red blood cell scanning has the great advantage that the threshold for detecting extravasation into the gut is only 20% of that required by angiography (31,54,55).
Two studies have used technetium scintigraphy, but the method was unsuccessful to show the bleeding site (53,54). However, there are several case reports that show great utility of scintigraphy in diagnosis and management of these cases, especially when endoscopy fails and surgery is at high risk (12,31). According to new data, Tc99m RBCs may accurately localize the site of bleeding in 88–97% of patients, with positive findings resulting in a 5-fold greater likelihood that the patient will require surgery (31,55).
Pharmacologic, Endoscopic and Surgical Therapy, Rate of Rebleeding, Mortality, Need for Surgery
There is currently no consensus on the treatment of Dieulafoy’s lesions. Treatment depends on the presentation, lesion site and mostly on site available endoscopic and surgical expertise (36,37). Bleeding can be fatal and treatment was mostly surgical before 1990 (2,5,7,9,38). Nowadays, endoscopic therapy has replaced surgery (36,37). The need for surgery has decreased and so has mortality since progress in endoscopic diagnosis and hemostasis (5,10).
Proton pump inhibitors remain a valuable therapeutic tool and according to current guidelines (36,37), they should be initiated as intravenous bolus as soon as an upper gastrointestinal bleeding is suspected. Though the effect of pre-endoscopic therapy is nonsignificant on mortality, rebleeding, surgery or blood transfusion, it reduces the incidence of stigmata of recent hemorrhage. Most studies and case series and reports in the literature used intravenous proton pump inhibitors infusion after endoscopic hemostasis (3,4,6,17,24,35). There is little data to suggest the efficacy of proton pump inhibitors before or after endoscopy particularly in Dieulafoy lesion as compared to other non-variceal etiologies of upper gastrointestinal bleeding (31,36,37).
Available endoscopic methods include: injection therapy, heater probe, bipolar electrocoagulation, laser therapy, hemoclips and rubber band ligation, Hemospray (36,37).
Adrenaline injection is easy, low cost and safe, but as a single hemostatic method it is associated with a relatively high rebleeding rate. The advantages of adrenalin injection are represented by several facts: it provides temporary hemostasis, enhances visualization of the hemorrhagic site without the risk of tissue injury and perforation (36,37,56,57).
Contact thermocoagulation has the risk of transmural injury and seems less effective than hemoclip placement (31,37,38,58). However, hemoclip placement may be difficult in the duodenum, sometimes acute duodenal angulation makes hemoclip placement impossible or very difficult. Hemoclip placement is effective especially if the surrounding mucosa is soft (8,34,59,60).
Band ligation seems easier than hemoclip placement, as well as safer due to lower perforation risk compared with thermal methods. The risks of rebleeding and ulcer formation around the ligated mucosa are low (61,62).
Studies have shown that mechanical endoscopic methods such as hemoclip and rubber band ligation are more effective than injection and thermal therapy. It has also been shown that the use of 2 combined endoscopic techniques is superior to using a single endoscopic method (36,37,56).
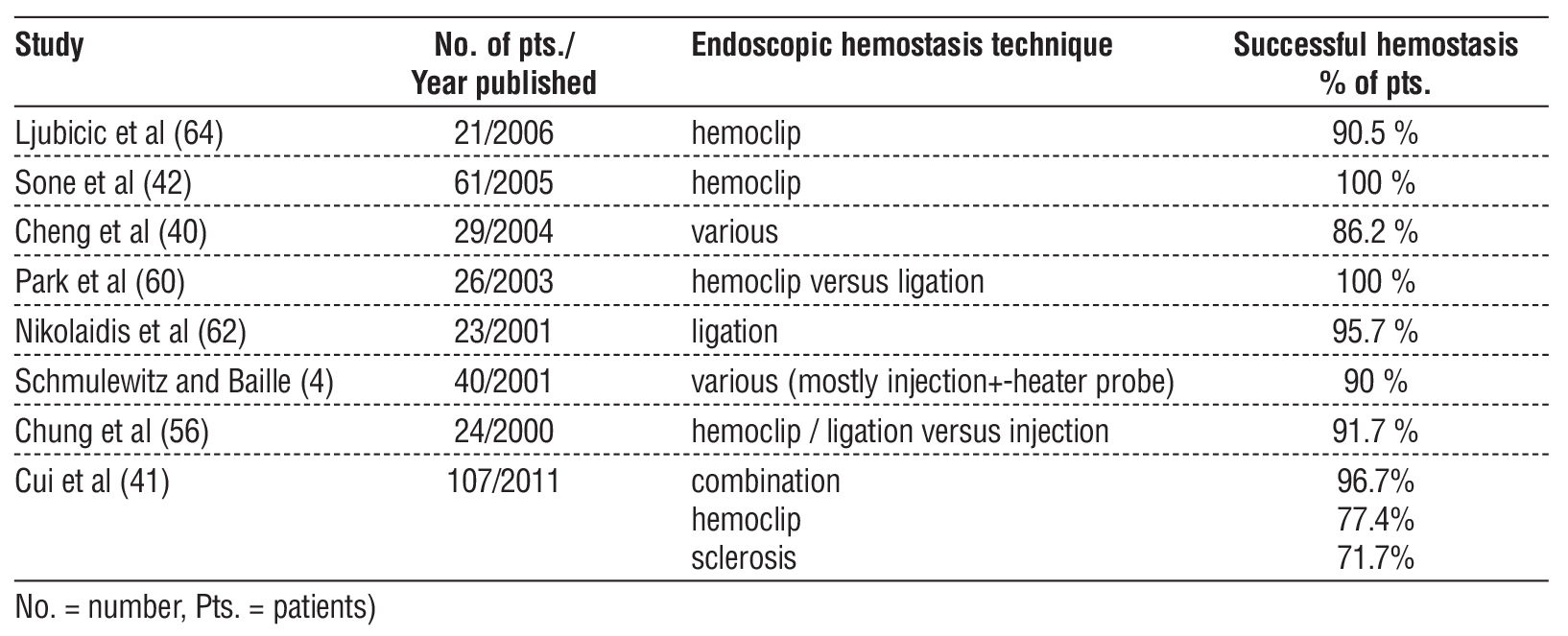
Risk of rebleeding was high and surgical treatment was more indicated in the past, but numbers have definitely become much better in the last endoscopic years, as seen in the table 1. Duodenal localization does not appear to have a higher risk for rebleeding (63).
Table 1 - Results of endoscopic hemostasis in different studies.
Another new endoscopic therapeutic tool are hemostatic powders, which seem quite attractive, as they are safe and technically much easier to apply than hemoclipping and/or band ligation (65,66).
When endoscopic therapy fails, alternative treatments include angiography and eventually surgical treatment (31,50). Angiography followed by embolization is indicated in poor surgical candidates in whom endoscopy has failed or localization is difficult to reach. A major disadvantage of angioembolization is the risk of ischemia due to collaterals feeding the abnormal vessel. Re-bleeding can be explained by collateral circulation or non-complete embolization of the feeding artery.
Up to date, there is enough evidence in the literature to justify selective arterial embolization as a less invasive alternative therapeutic option. However, to the best of our knowledge, no study has established its role as the first initial treatment (50,51).
The rate for surgical necessity has decreased from 100% to 3% with increasing endoscopic and surgical expertise (57). The bleeding source can be sutured with a 3-point U stitch technique.
The normal surrounding mucosa makes it impossible to feel the lesion, so frequently pre- or intra-operative endoscopy is needed (31,57). Hemoclip placement can be useful for guiding the surgeon even if endoscopic hemostasis fails (6,60). Main indications for surgical treatment are: hemodynamic instability or failure of other methods (31,41).
Initially described as a necroptic study by Dieulafoy, nowadays early endoscopic treatment has decreased mortality rates by up to 8% (5,42). Mortality rates are mostly associated with comorbidities such as organ failure or sepsis (3,33). Previous studies with lower rates of endoscopic treatments show relatively higher mortality rates ranging between 23-35% (31,42).
CONCLUSIONS
We find our case very representative for the fact that mucosal lesion was minimal, adjacent mucosa was perfectly normal (no inflammation, exudate or ecchymosis) with a minimal Forrest IB hemorrhagic pinpoint. The case also underlines the importance of urgent endoscopy in selected cases, delay being strongly associated with the risk of impossibility to identify the Dieulafoy lesion localization once the bleeding has stopped.
The historical lesion is rare, subtle and possibly difficult to recognize (especially in delayed endoscopy) but it remains a potentially life-threatening condition mostly in patients with multiple comorbidities, such as our patient with chronic renal failure and hemodialysis. Urgent endoscopy remains a very useful diagnosis and life saving therapeutic tool, especially in patients with multiple comorbidities and high surgical risk. The specific treatment of choice should be individualized to each patients’ clinical situation (hemodynamic stability, comorbidities) as well as technical expertise of the endoscopist and surgeon.
We think that delayed endoscopy in such cases could be responsible for the well-known” iceberg effect” but also the so called Baader-Meinhof phenomenon (68), which refers to the cognitive bias when one has become aware of an entity through a new experience or learning and then begins to encounter and recognize it more frequently, even if it has been there all along.
Conflicts of Interest: none to declare.
Ethical Statement
All procedures performed in this study were in accordance with the ethical standards of the
institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013).
REFERENCES
1. Saleh R, Lucerna A, Espinosa J, Scali V. Dieulafoy lesion: the little known sleeping giant of gastrointestinal bleeds. Am J Emerg Med. 2016;34(12):2464.e3-2464.e5.
2. Gallard T. Aneurysmes miliaires de l’estomac donnant lieu à des hématémèses mortelles. Bulletins et Mémoires de la Société Médicale des Hôpitaux de Paris, 1884.
3. Nguyen D, Jackson CS. The Dieulafoy’s Lesion. An Update on Evaluatioan,Diagnosis, and Management. J Clin Gastroenterol. 2015;49(7):541-9.
4. Schmulewitz N, Baillie J. Dieulafoy lesions: a review of 6 years of experience at a tertiary referral center. Am J Gastroenterol. 2001; 96(6):1688-94.
5. Senger JL, Kanthn R. The Evolution of Dieulafoy’s Lesion Since 1897: Then and Now-A Journey through the Lens of a Pediatric Lesion with Literatura Review. Gastroenterol Res Pract. 2012; 2012:432517.
6. López-Arce G, Zepeda-Gómez S, Chávez-Tapia NC, Garcia-Osogobio S, Franco-Guzmán AM, Ramirez-Luna MA, et al. Upper gastro-intestinal Dieulafoy’s lesions and endoscopic treatment: first report from a mexican centre. Therap Adv Gastroenterol. 2008;1(2):97-101.
7. Baettig B, Haecki W, Lammer F, Jost R. Dieulafoy's disease: endoscopic treatment and follow up. Gut. 1993;34(10):1418-21.
8. Clements J, Clements B, Loughrey M. Gastric Dieulafoy lesion: a rare cause of massive haematemesis in an elderly woman. BMJ Case Rep. 2018;2018:bcr2017223615.
9. Veldhuyzen van Zanten Z, Schipper B, Tytga M. Recurrent massive haematemesis from Dieulafoy vascular malformations-a review of 101 cases. Gut. 1986;27(2):213-22.
10. Amarnath S, Ghimire S, Khan HM. (2020). A Tale of Three Dieulafoy Lesions: A Case Report and Review of the Literature. Cureus. 2020;12(5):e8365.
11. Malliaras GP, Carollo A, Bogen G. Esophageal Dieulafoy’s lesion: an exceedingly rare cause of massive upper GI bleeding. J Surg Case Rep. 2016;2016(6):rjw074.
12. Salles AVJ, Resende RB, Seiji G, Coaglio RC. Dieulafoy’s Lesion Associated with Megaesophagus. Arch Clin Gastroenterol. 2020; 6(3):067-068.
13. Alye?il C, Özturan ?U, Do?an Ö. Dieulafoy’s Lesion:A Rare Location for Gastrointestinal Hemorrhage. J Emerg Med Case Rep. 2017;8: 62-3.
14. Dirweesh A, Chikezie A, Khan YM, Zia S, Tahir M. Postural Syncope and Constipation:An Unusual Presentation of a Duodenal Dieulafoy’s Lesion. Case Rep Gastrointest Med. 2017;2017:6983434.
15. He ZW, Zhong L, Xu H, Shi H, Wang YM, Liu XC. Massive gastro-intestinal bleeding caused by a Dieulafoy’s lesion in a duodenal diverticulum: A case report. World J Clin Cases. 2020;8(20):5013-5018.
16. Hernandez G, Fuertes Y, Casamayor N. A Case of Duodenal Dieulafoy’s Lesion. CHEST Annual Meeting. 2019;2204A.
17. Inayat F, Amjad W, Hussain Q, Hurairah A. Dieulafoy’s lesion of the duodenum: a comparative review of 37 cases. BMJ Case Rep. 2018; 2018:bcr2017223246.
18. Lee HH, Jeong SM, Kim GM, Ku JK. Laparoscopic Resection of Bleeding Dieulafoy’s Lesion in the Jejunum Following Intraoperative Localization Using Endoscopy. J Minim Invasive Surg 2016;19(1): 39-42
19. Murali U, Ahmad M, Hali A, Hamidin M. (2017).Melena Secondary to Duodenal Diuulafoy’s Lesion:A Rare Case Report. Journal of Clinical and Diagnostic Research. 2017;11(11):PD06-PD08.
20. Oladunjoye O, Oladunjoye A, Slater L, Jehangir A. Dieulafoy lesion in the jejunum: a rare cause of massive gastrointestinal bleeding. J Community Hosp Intern Med Perspect. 2020;10(2):138-139.
21. Paikos D, Moschos J, Sofianidis J, Zavos C, Tikos G, Theodoridis K, Dimakopoulou,A., Mpakas,P.,Raptis,D.,Papaziogas, B. Dieulafoy’s Lesion of Ileum:Report of A Rare Case. Arch Clin Med Case Rep. 2020;4(6):1140-1145.
22. Pessia B, Romano L, Giuliani A, Gizzonio D, Schietroma M, Carla F, et al. Rare case of upper gastrointestinal bleeding: Dieulafoy’s lesion of duodenum. A case report. Ann Med Surg (Lond). 2019;45:19-21.
23. Saadaa M, Pereka S, Agbariaa M, Pasteura AR. Massive Gastro-intestinal Bleeding from a Jejunal Dieulafoy Lesion: An Extraordinary Presentation. Case Rep Gastroenterol. 2019;13(3):508-513.
24. Rojas A, Carvajal DG, Prieto RG, Aponte DM. An Unusual Finding of a Dieulafoy’s Lesion in the Duodenum. Rev Col Gastroenterol. 2016;31(3):287-291.
25. Inayat F, Ullah W, Hussain Q, Abdullah, MA. Dieulafoy’s lesion of the colon and rectum: a case series and literature review. BMJ Case Rep. 2017;2017:bcr2017220431.
26. Omar E, Rani T, Andrew B, Ofosub A, Prashanth O, Rawlac P, et al. Rectal Dieulafoy’s Lesion: A Rare Etiology of Lower Gastrointestinal Hemorrhage. Case Rep Gastroenterol. 2019;13(1):73-77.
27. Yang D, Rong C, Gu J, Ma M, Xu L, Da M, et al. Dieulafoy disease of the trachea with recurrent episodes of massive hemoptysis. Medicine (Baltimore). 2017;96(5):e5855.
28. Qian X, Du Q, Wei N, Wang M, Wang H, Tang Y. Bronchial Dieulafoy’s disease: a retrospective analysis of 73 cases. BMC Pulm Med. 2019;19(1):104.
29. Liao SX, Sun PP, Li BG, He SF, Liu MM, Yang YO. A rare and fatal respiratory disease: bronchial Dieulafoy’s disease. Ther Adv Respir Dis. 2020;14:1-5.
30. Chena W, Chenb M, Lib X, Gaob X, Lib J. Clinical characteristics and treatments for bronchial Dieulafoy's disease. Respir Med Case Rep. 2019;26:229-235.
31. Baxter M, Aly EH. Dieualafoy’s lesion: currrent trends in diagnosis and management. Ann R Coll Surg Engl. 2010;92(7):548-54.
32. Massinha P, Cunha I, Tome L. Dieulafoy Lesion:Predictive Factors of Early Relapse and Long-Term Follow-Up. GE Port J Gastroenterol. 2020;27(4):237-243.
33. Shin HJ, Ju JS, Kim KD, Kim SW, Kang SK, Kang SH, et al. Risk Factors for Dieulafoy Lesions in the Upper Gastrointestinal Tract. Clin Endosc. 2015;48(3):228-33.
34. Malik F, Salman OA, Alchalabi M, Chaudhari S, Khan AT. Dieulafoy lesions as cause of upper gastrointestinal bleeding in a patient with portal hypertension. J Community Hosp Intern Med Perspect. 2021; 11(1):94-95.
35. Nardo G, Esposito G, Mauro A, Zenzeri L, Ciccarelli GP, Catzola A, Rossi A, et al. Dieulafoy lesion: two pediatric case reports. Ital J Pediatr. 2020;46(1):48.
36. Gralnek IM, Stanley AJ, Morris AJ, Camus M, Lau J, Lanas A, et al. Endoscopic diagnosis and management of nonvariceal upper
gastrointestinal hemorrhage (NVUGIH): European Society of Gastrointestinal Endoscopy (ESGE) Guideline – Update 2021. Endoscopy. 2021;53(3):300-332.
37. Laine L, Barkun AN, Saltzman JR, Martel M, Leontiadis GI. ACG Clinical Guideline: Upper Gastrointestinal and Ulcer Bleeding. Am J Gastroenterol. 2021;116(5):899-917.
38. McClave SA, Goldschmid S, Cunningham JT. Dieulafoy’s cirsoid aneurysm of the duodenum. Dig Dis Sci. 1988;33(7):801-5.
39. Wu JM, Zaitoun AM. A galling disease? Dieulafoy’s lesion of the
gallbladder. Int J Surg Case Rep. 2018:44:62-65.
40. Cheng CL, Liu NJ, Lee CS, Chen PC, Ho YP, Tang JH, et al. Endoscopic management of Dieulafoy lesions in acute nonvariceal upper gastrointestinal bleeding. Dig Dis Sci. 2004;49(7-8):1139-44.
41. Cui J, Huang LY, Liu YX, Song B, Yi LZ, Xu N, et al. Efficacy of endoscopic therapy for gastrointestinal bleeding from Dieulafoy's lesion. World J Gastroenterol. 2011;17(10):1368-72.
42. Sone Y, Kumada T, Toyoda H, Hisanaga Y, Kiriyama S, Tanikawa M. Endoscopic management and follow up of Dieulafoy lesion in the upper gastrointestinal tract. Endoscopy. 2005;37(5):449-53.
43. Yano T, Yamamoto H, Sunada K, Miyata T, Iwamoto M, Hayashi Y, et al. Endoscopic classification of vascular lesions of the small intestine (with videos). Gastrointest Endosc. 2008;67(1):169-72.
44. Sakai E, Ohata K, Nakajima A, Matsuhashi L. Diagnosis and therapeutic strategies for small bowel vascular lesions. World J Gastroenterol. 2019;25(22):2720-2733.
45. Nojkov B, Cappell MS. Gastrointestinal bleeding from Dieulafoy's lesion: Clinical presentation, endoscopic findings, and endoscopic therapy. World J Gastrointest Endosc. 2015;7(4):295-307.
46. Jeon HK, Kim GH. Endoscopic Management of Dieulafoy's Lesion. Clin Endosc. 2015;48(2):112-20.
47. Ciobanu L, Pascu O, Diaconu B, Matei D, Pojoga C, Tanåãu M. Bleeding Dieulafoy's-like lesions of the gut identified by capsule endoscopy. World J Gastroenterol. 2013;19(29):4823-6.
48. Maeda Y, Moribata K, Deguchi H, Inoue I, Maekita T, Iguchi M, et al. Video capsule endoscopy as the initial examination for overt obscure gastrointestinal bleeding can efficiently identify patients who require double-balloon enteroscopy. BMC Gastroenterol. 2015;15:132.
49. Khalid S, Abbass A, Do T, Malhotra D, Albors-Mora M. The Hidden Culprit in a Massive Episode of Hematemesis: A Dieulafoy's Lesion. Cureus. 2016;8(10):e824.
50. Kwon JH, Kim JS. Transcatheter Arterial Embolisation of Acute Nonvariceal Upper Gastrointestinal Bleeding Refractory to Endoscopic Haemostasis. Hong Kong J Radiol. 2020;23:164-75.
51. Mohd Rizal MY, Kosai NR, Sutton PS, Rozman Z, Razman J, Harunarashid H, et al. Arterial Embolization of a Bleeding Gastric Dieulafoy Lesion: A Case Report. Clin Ter. 2013;164(1):25-7.
52. Mohamed AB, Mohamed SMM. Combined Management Approach for Gastric & Extra-Gastric Dieulafoy’s Lesions. Adv Res Gastroentero Hepatol. 2018;10(5):555800.
53. Sai Prasad TR, Lim HL, Lim KH, Yap TL. Bleeding jejunal dieulafoy pseudopolyp: capsule endoscopic and laparoscopic-assisted resection. J Laparoendosc Adv Surg Tech A. 2007;17(4):509-12.
54. Goins WA, Chatman DM, Kaviani MJ. Massive lower gastrointestinal bleeding due to ‘Dieulafoy’s vascular malformation’ of the jejunum: case report. J Natl Med Assoc. 1995;87(10):766-70.
55. Nojkov B, Cappell MS. Gastrointestinal bleeding from Dieulafoy's lesion: Clinical presentation, endoscopic findings, and endoscopic therapy. World J Gastrointest Endosc. 2015;7(4):295-307.
56. Chung IK, Kim EJ, Lee MS, Kim HS, Park SH, Lee MH, et al. Bleeding Dieulafoy's lesions and the choice of endoscopic method: comparing the hemostatic efficacy of mechanical and injection methods. Gastrointest Endosc. 2000;52(6):721-4.
57. Yilmaz M, Ozütemiz O, Karasu Z, Ersöz G, Günsar F, Batur Y, et al. Endoscopic injection therapy of bleeding Dieulafoy lesion of the stomach. Hepatogastroenterology. 2005;52(65):1622-5.
58. Zavras N, Siafakas C, Pergamalis G, Verney Y, Clavdianou M, Salakos C. Successful diagnosis and treatment of Dieulafoy's lesion with endoscopy and thermocoagulation in a full-term neonate: Report of a case and literature review. J Ped Surg Case Reports. 2014;2(5):250-253.
59. Jung K, Moon W. Role of endoscopy in acute gastrointestinal bleeding in real clinical practice: An evidence-based review. World J Gastrointest Endosc. 2019;11(2):68-83.
60. Park CH, Sohn YH, Lee WS, Joo YE, Choi SK, Rew JS, et al. The usefulness of endoscopic hemoclipping for bleeding Dieulafoy lesions. Endoscopy. 2003;35(5):388-92.
61. Barakat M, Hamed A, Shady A, Homsi M, Eskaros S. Endoscopic band ligation versus endoscopic hemoclip placement for Dieulafoy's lesion: a meta-analysis. Eur J Gastroenterol Hepatol. 2018;30(9): 995-996.
62. Nikolaidis N, Zezos P, Giouleme O, Budas K, Marakis G, Paroutoglou G, et al. Endoscopic band ligation of Dieulafoy-like lesions in the upper gastrointestinal tract. Endoscopy. 2001;33(9):754-60.
63. Lai Y, Rong J, Zhu Z, Liao W, Li B, Zhu Y, et al. Risk Factors for Rebleeding after Emergency Endoscopic Treatment of Dieulafoy Lesion. Can J Gastroenterol Hepatol. 2020;2020:2385214.
64. Ljubicic N. Efficacy of endoscopic clipping and long-term follow-up of bleeding Dieulafoy's lesions in the upper gastrointestinal tract. Hepatogastroenterology. 2006;53(68):224-7.
65. Aziz M, Weissman S, Mehta TI, Hassan S, Khan Z, Fatima R, et al.: Efficacy of hemospray in non-variceal upper gastrointestinal bleeding: a systematic review with meta-analysis. Ann Gastroenterol. 2020;33(2):145-154.
66. Mourad FH, Leong RW. Role of hemostatic powders in the management of lower gastrointestinal bleeding: A review. J Gastroenterol Hepatol. 2018;33(8):1445-1453.
67. Pessia B, Romano L, Giuliani A, Domenico G, Mario S, Francesco C, et al. Rare case of upper gastrointestinal bleeding: Dieulafoy’ s lesion of duodenum. A case report. Ann Med Surg (Lond). 2019;45:19-21.
68. Kolli S, Dang-Ho KP, Mori A, Gurram K. The Baader-Meinhof Phenomenon of Dieulafoy’s Lesion. Cureus. 2019;11(5):e4595.
Full Text Sources:
Abstract:
Views: 10

For Authors
Journal Subscriptions

Sept 2025
Supplements
Instructions for authors
Online submission
Contact
e-ISSN: 2601 - 1700 (online)
ISSN-L: 2559 - 723X
Journal Abbreviation: Surg. Gastroenterol. Oncol.
Surgery, Gastroenterology and Oncology (SGO) is indexed in:
- SCOPUS
- EBSCO
- DOI/Crossref
- Google Scholar
- SCImago
- Harvard Library
- Open Academic Journals Index (OAJI)
Surgery, Gastroenterology and Oncology (SGO) is an open-access, peer-reviewed online journal published by Celsius Publishing House. The journal allows readers to read, download, copy, distribute, print, search, or link to the full text of its articles.
Time to first editorial decision: 25 days
Rejection rate: 61%
CiteScore: 0.2

Meetings and Courses in 2025
Meetings and Courses in 2024
Meetings and Courses in 2023
Meetings and Courses in 2022
Meetings and Courses in 2021
Meetings and Courses in 2020
Meetings and Courses in 2019
Verona expert meeting 2019

Surgery, Gastroenterology and Oncology applies the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits readers to copy and redistribute the material in any medium or format, remix, adapt, build upon the published works non-commercially, and license the derivative works on different terms, provided the original material is properly cited and the use is non-commercial. Please see: https://creativecommons.org/licenses/by-nc/4.0/
Publisher’s Note:
The opinions, statements, and data contained in article are solely those of the authors and not of Surgery, Gastroenterology and Oncology journal or the editors. Publisher and the editors disclaim responsibility for any damage resulting from any ideas, instructions, methods, or products referred to in the content.
 IASGO Society News
IASGO Society News