Surgery, Gastroenterology and Oncology
|
|
ABSTRACT
Background: One of the most crucial elements in assessing breast cancer prognosis is axillary lymph node status. While ultrasonography (US) is routinely used for pre-therapeutic axillary evaluation, its diagnostic performance post-neoadjuvant chemotherapy (NAC) requires further validation. Objective: To evaluate the reliability and accuracy of ultrasound assessment of axillary lymph nodes before and after neoadjuvant chemotherapy, comparing it with sentinel lymph node biopsy and final paraffin histopathological findings in post-neo-adjuvant axillary surgical management of early-stage female breast cancer.
Methods: This prospective cross-sectional study included 40 female patients with early breast cancer (cT1-T2) between February 2023 and August 2024. Patients underwent axillary US assessment before and after NAC. Node-positive patients (cN1) had US-guided clip placement pre-NAC. Post-NAC management included sentinel lymph node biopsy (SLNB) or axillary lymph node dissection (ALND) with results compared to final histopathology.
Results: Pre-NAC axillary US identified 26 (65%) clinically node-negative (cN0) and 14 (35%) node-positive (cN1) cases. Post-NAC, 36 (90%) converted to ycN0 status, while 4 (10%) remained ycN1. Of the 36 SLNB procedures, 31 (86.1%) were histopathologically negative. In the final histopathological analysis, 77.7% of axillary lymph node dissection (ALND) cases were positive. Similarly, 71.5% of clipped nodes tested positive. Post-NAC US demonstrated 94% specificity, 82.5% accuracy, and 86.1% negative predictive value, though sensitivity was 28.6%. Pre-NAC US showed higher sensitivity (66.7%) with 85.5% accuracy.
Conclusion: Axillary US shows high specificity and negative predictive value in post-NAC nodal assessment, making it particularly valuable for identifying patients who achieve nodal complete response. The high conversion rate to ycN0 (90%) supports its utility in monitoring treatment response and surgical planning.
INTRODUCTION
One of the most crucial elements in assessing prognosis and guiding treatment in breast cancer is the status of axillary lymph nodes (1). Ultrasonography (US) has become a routine preoperative diagnostic tool for pre-therapeutic axillary evaluation, with recent studies demonstrating its value in initial staging (2,3). However, its accuracy in assessing nodal status after neoadjuvant chemotherapy (NAC) remains an area of active investigation (4).
Multiple trials have demonstrated the feasibility of sentinel lymph node biopsy (SLNB) after NAC, though with false-negative rates exceeding 10% (5). The American College of Surgeons Oncology Group Z1071 (Alliance) trial showed that removing the clipped positive lymph node during SLNB could reduce this false-negative rate to 6.8% (6). Patients can generally be classified into three groups based on their histological nodal response before and after neoadjuvant chemotherapy (NAC): (1) cN0 patients who remain ycN0, (2) cN+ patients who remain ycN+, and (3) cN+ patients who convert to ycN0 following NAC. Identifying these groups is crucial for determining the most appropriate axillary management approach.
Due to limited diagnostic accuracy of ultrasonography in determining post-NAC nodal status (ycN), with approximately 50% of sonographically negative patients showing residual axillary disease in surgical specimens, the key diagnostic challenge lies in accurately identifying true nodal responders (7,8).
Although sentinel lymph node biopsy (SLNB) has a false-negative rate exceeding 10% in patients with cN+ disease, studies have shown that this rate can be significantly lowered by retrieving at least three sentinel lymph nodes (FNR ~8%) or by incorporating targeted axillary dissection (TAD) with removal of the clipped node (FNR <4%).
The increasing use of NAC in early-stage breast cancer, combined with the potential to avoid axillary lymph node dissection in complete responders, underscores the importance of reliable post-treatment nodal assessment (9,10). The Alliance A011202 trial (NCT01901094) is currently investigating whether extended nodal radiation could be a viable alternative to axillary lymph node dissection (ALND) in patients with residual nodal disease (ypN+). Although promising, this approach remains experimental until more conclusive evidence is obtained.
Recent studies have shown nodal response rates of 40-75% after NAC, highlighting the potential for surgical de-escalation in selected patients (11,12). Given the limited diagnostic accuracy of ultrasonography in assessing post-NAC nodal status (ycN), research indicates that nearly 50% of patients with sonographically negative nodes (ycN0) still have residual axillary disease upon histopathological evaluation. This underscores the importance of histopathological confirmation through SLNB or ALND to ensure precise staging and optimal surgical planning.
This study aims to evaluate the reliability and accuracy of ultrasound assessment of axillary nodes before and after NAC, correlating findings with sentinel lymph node biopsy results and final histopathological analysis in patients with early breast cancer.
Figure 1 - Intraoperative specimen of a retrieved clipped node (ycN0) sent for radiological confirmation following wire localization.

Materials and Methods
Study Design and Ethics
This prospective cross-sectional analytic study was conducted at Al Kasr-Al Ainy Teaching Hospital, Cairo University Hospitals, from February 2023 to August 2024. The study protocol was approved by the Ethics Committee of the Faculty of Medicine, Cairo University (approval number MD_72_2023). Written informed consent was obtained from all participants before enrollment.
Study Population and Sample Size
Sample size was calculated using G*Power software. Based on the primary outcome (accuracy of axillary ultrasound as diagnostic tool pre and post neoadjuvant chemotherapy), 44 patients were determined appropriate with 80% power and ? error probability of 0.05, effect size of 0.21. Forty patients completed the study after excluding four cases due to disease progression during NAC.
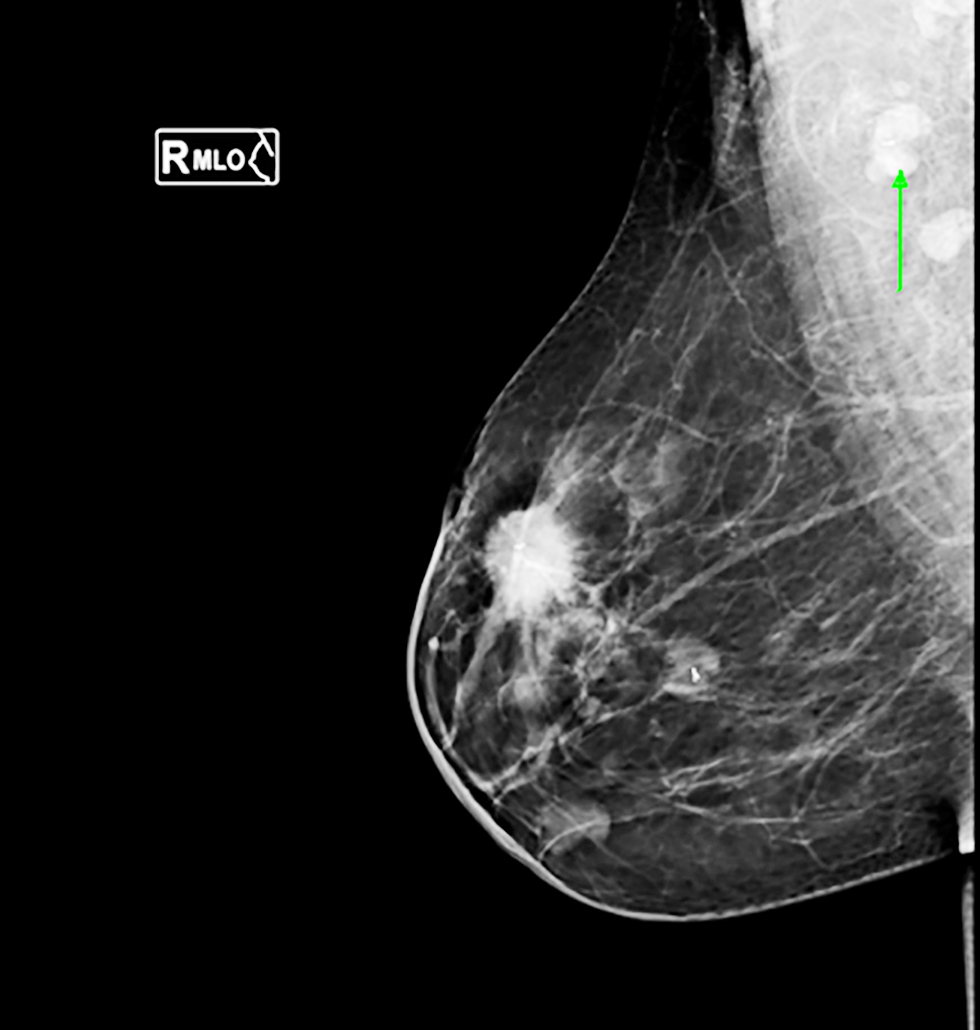
Figure 2 - Mammogram imaging showing ultrasound-guided clip placement of a pathological right axillary lymph node in a triple-negative breast cancer case
Patient Selection
Inclusion criteria:
· Female patients with early breast cancer (cT1-T2);
· Clinical nodal status cN0-cN1;
· Candidates for neoadjuvant chemotherapy;
· Breast cancer subtypes: triple-negative, triple-positive, or HER2-enriched.
Exclusion criteria:
· Disease progression during NAC;
· Medically unfit for surgery;
· Previous axillary surgery including FNAC or SLNB;
· Locally advanced breast cancer (cT3-4, cN2-3);
· Metastatic disease;
· Recurrent breast cancer.
Study Procedures
Clinical assessment
· All patients underwent thorough clinical examination including bilateral breast examination and regional lymph node assessment. Initial workup included complete laboratory investigations, ECG, and echocardiography. Metastatic workup was performed according to clinical stage (13).
Imaging protocol
· Bilateral breast sono-mammography with focused axillary ultrasound was performed using high-frequency linear transducer (9-12 MHz).
· Axillary lymph nodes were evaluated for:
¨ Size (long and short axis);
¨ Shape (L/S ratio);
¨ Cortical thickness;
¨ Hilum characteristics.
· US-guided core biopsy for primary tumor and suspicious nodes (14).
· MRI with contrast or contrast-enhanced spectral mammography when indicated for surgical planning.
Node marking technique
For cN1 patients, suspicious nodes underwent US-guided clip placement pre-NAC using titanium clips. Post-NAC, patients with clipped nodes underwent wire localization before surgery under US guidance (15).
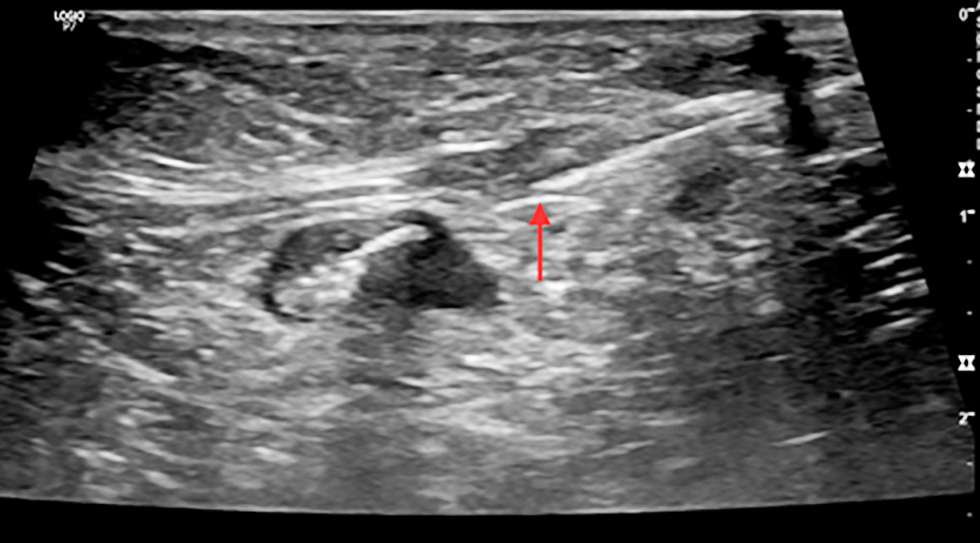
Figure 3 - Ultrasound image showing clip insertion with needle in a pathological axillary lymph node (cN1) in a female with early breast cancer
Surgical Procedures
Patients were categorized into two groups:
· Group I (cN0): Underwent SLNB post-NAC;
· Group II (cN1): Underwent targeted axillary dissection with clipped node identification.
SLNB was performed using patent blue dye with a minimum of three nodes harvested. Intraoperative specimen radiography was performed for clipped nodes to confirm retrieval. Management followed ACOSOG Z0011 criteria (16):
· Negative SLNB: No further surgery;
· 1-2 positive nodes: No further surgery if meeting Z0011 criteria;
· ?3 positive nodes or extracapsular extension: Completion ALND;
· Patients with isolated tumor cells (ITCs): no additional ALND is required;
· Patients with micrometastases or macrometastases (1-2 positive lymph nodes out of 3-4
examined): No further ALND is needed.
The lymph node was submitted for paraffin section assessment to reassess the frozen section results, with management modified accordingly. An example of retrieved clipped lymph node is shown in fig. 1, while clip placement procedures are illustrated in figs. 2, 3, and 4.
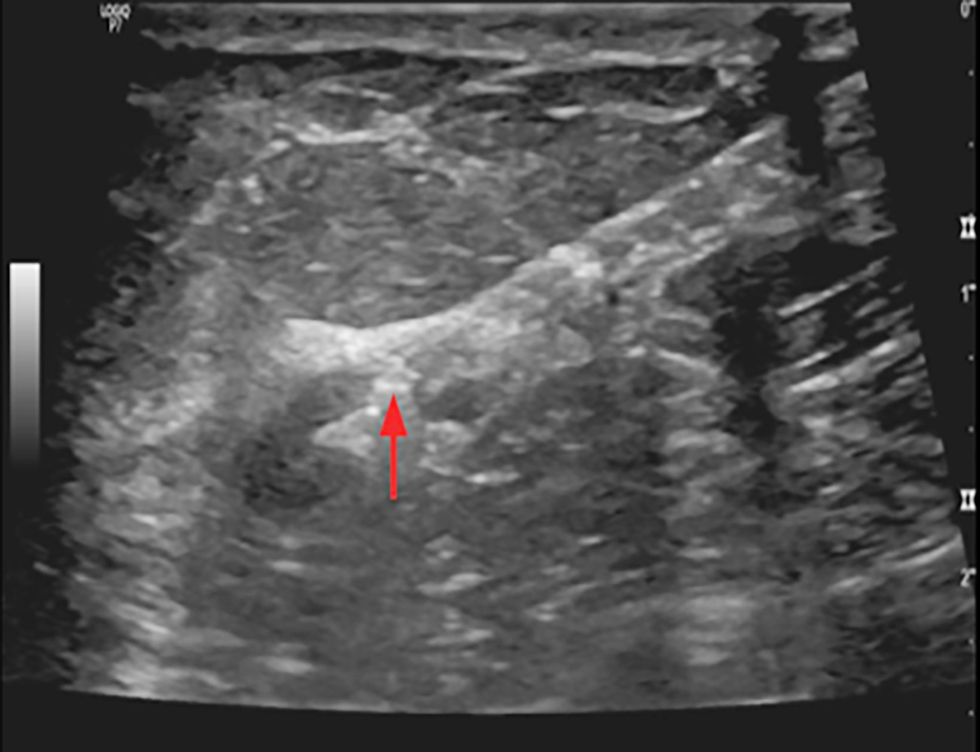
Figure 4 - Ultrasound imaging shows clip inserted in cortex of pathological axillary LN in a female with early breast cancer CN1
Pathological Assessment
All specimens underwent standard histopathological examination including:
· H&E staining;
· Analysis of retrieved sentinel nodes;
· Documentation of total number of nodes and number of positive nodes;
· Assessment of extracapsular extension;
· Complete ALND specimen evaluation when performed.
Secondary outcomes:
· Prevent unnecessary ALND and its associated complication, such as limb brawny edema.
Statistical Analysis
Data analysis was performed using SPSS version 27 (IBM Corp., Armonk, NY). Normality was assessed using Shapiro-Wilks test and histograms. Quantitative parametric data were presented as mean and standard deviation, analyzed using unpaired student t-test. Qualitative variables were presented as frequency and percentage, analyzed using Chi-square test or Fisher's exact test when appropriate. Diagnostic performance measures included sensitivity, specificity, positive and negative predictive values, and accuracy. P-value <0.05 was considered statistically significant (17).
RESULTS
This study analyzed 40 female patients with early-stage breast cancer who underwent axillary ultrasound (US) assessment pre- and post-neoadjuvant chemo-therapy (NAC). Four cases were excluded due to disease progression.
Patient and Tumor Characteristics
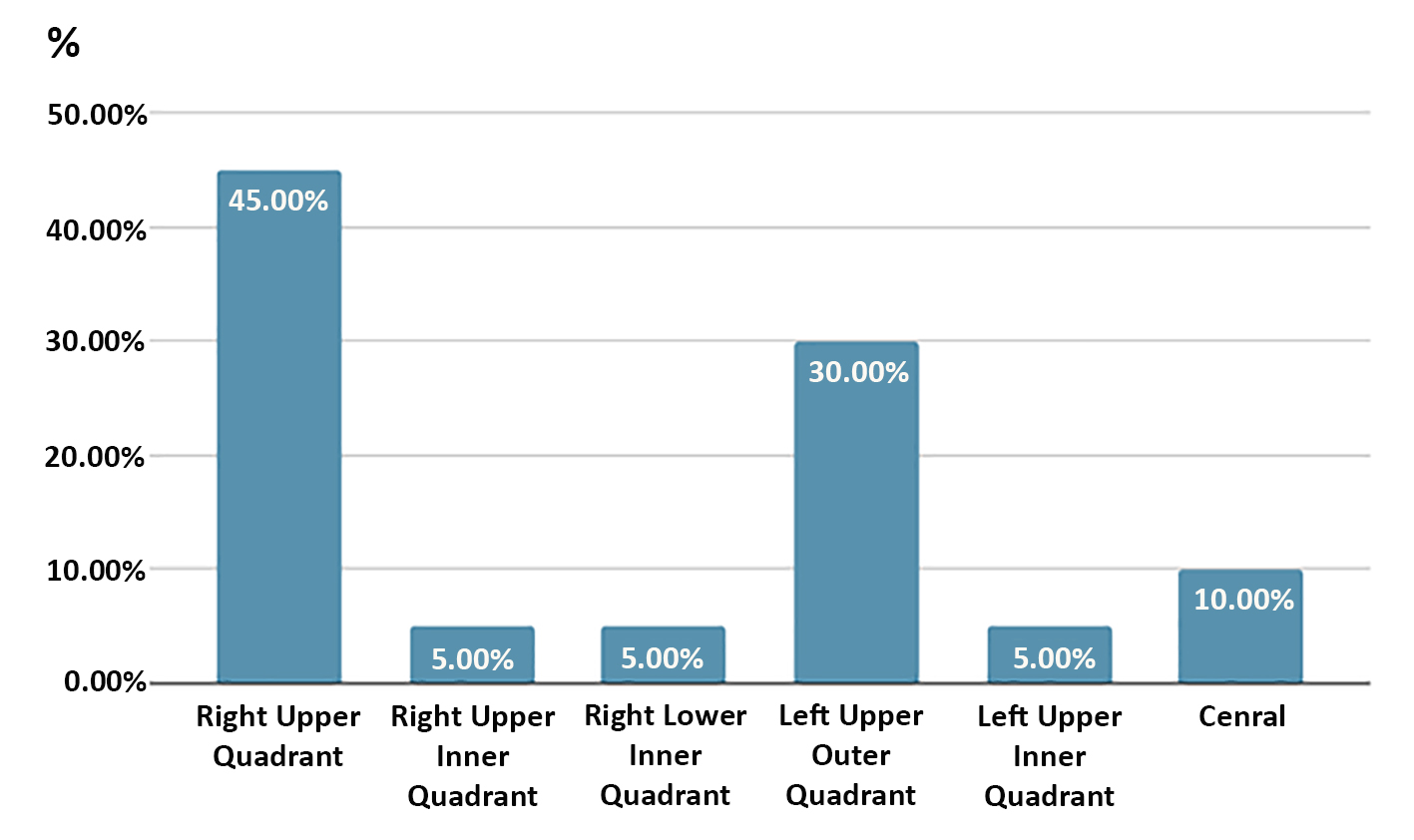
The mean age of patients was 57.62 ± 8.35 years (range 45-75 years). Primary tumor location was predominantly in the right upper outer quadrant (45%, n=18) and left upper outer quadrant (30%, n=12), with remaining tumors distributed among other quadrants (25%). The distribution of tumor sites is shown in fig. 5.
Figure 5 - Site of studied cases
Immunohistochemical analysis revealed that 42.5% (n=17) of tumors were HER2-enriched, 35% (n=14) were triple-negative, and 22.5% (n=9) were triple-positive. Immunohistochemical subtypes are illustrated in fig. 6.
Figure 6 - IHC4 of studied cases

Table 1 - Baseline Characteristics of the Study Population (n = 40)
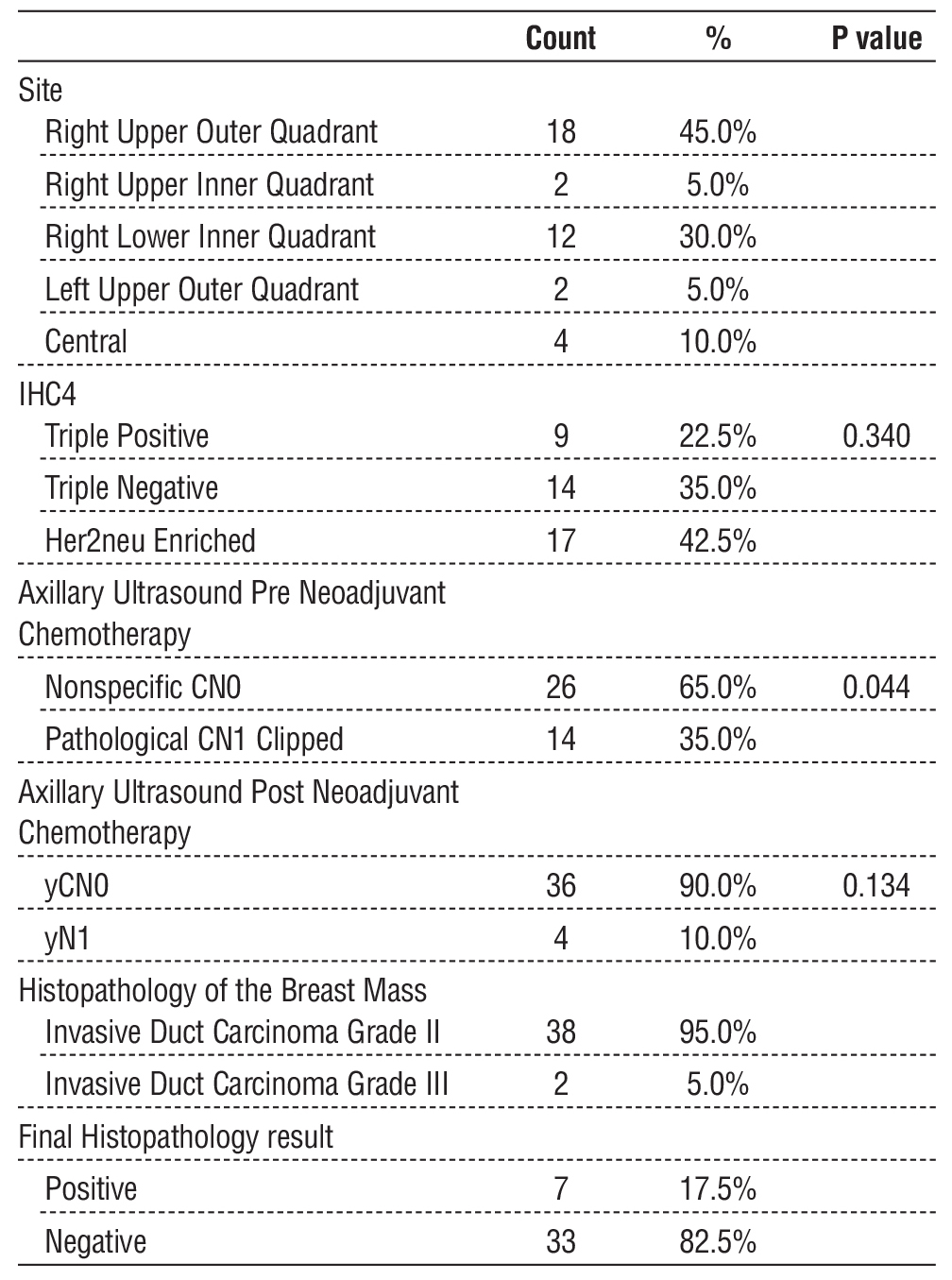
Histologically, 95% (n=38) of tumors were grade II invasive ductal carcinoma, while 5% (n=2) were grade III. These baseline tumor characteristics are summarized in table 1.
Axillary Ultrasound Findings Pre- and Post-NAC
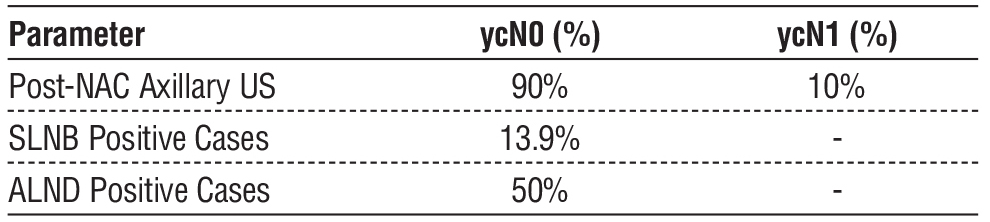
Before NAC, axillary US identified 26 patients (65%) as clinically node-negative (cN0) and 14 patients (35%) as node-positive (cN1), requiring clip placement. Following NAC, 36 patients (90%) converted to ycN0, while 4 patients (10%) remained ycN1. The pre- and post-NAC axillary ultrasound results are presented in tables 2 and 3, respectively. Axillary ultrasound features pre-NAC are visualized in fig. 7.
Surgical Outcomes
Among the 36 patients who underwent sentinel lymph node biopsy (SLNB), the mean number of retrieved nodes was 4.5 (range 3-6 nodes). Of these, 31 patients (86.1%) were histopathologically negative, while 5 (13.9%) were positive. Among clipped nodes (n=14), 10 cases converted to ycN0 and underwent SLNB, with 2 cases testing positive in final histopathology. The remaining 4 ycN1 cases underwent ALND, with 2 cases testing positive. Overall, 7 patients (17.5%) had positive nodes in final histopathology, while 33 patients (82.5%) were negative. Histopathological and clinical features are summarized in table 4.
Figure 7 - Axillary ultrasound pre-neoadjuvant chemotherapy of studied cases

Diagnostic Performance of Axillary Ultrasound
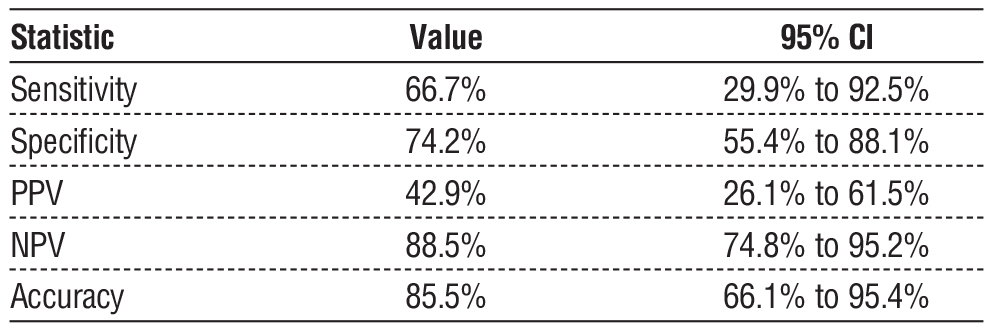
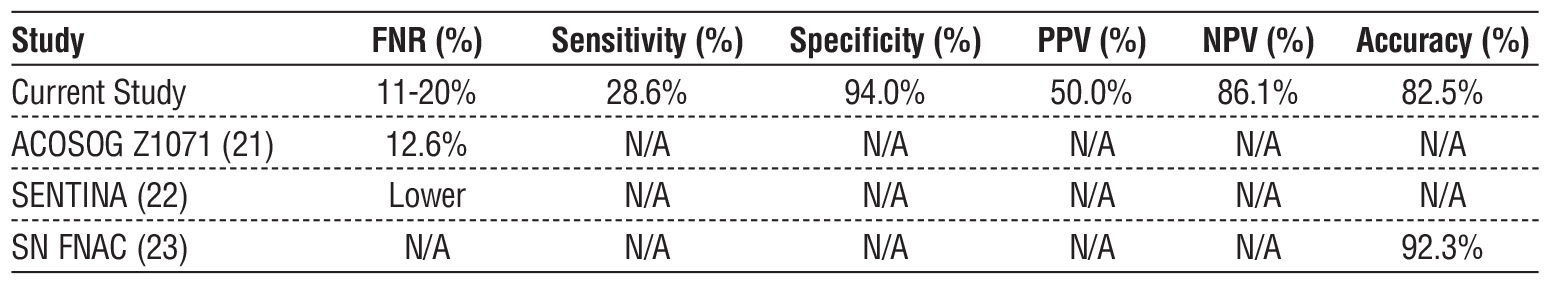
Pre-NAC US demonstrated a sensitivity of 66.7% (95% CI: 29.9% - 92.5%) and a specificity of 74.2% (95% CI: 55.4% - 88.1%), with an overall accuracy of 85.5% (95% CI: 66.1% - 95.4%) (1). Post-NAC US demonstrated a lower sensitivity of 28.6% (95% CI: 3.7% - 71.0%) but a high specificity of 94.0% (95% CI: 78.6% - 99.2%), with an overall accuracy of 82.5% (95% CI: 61.6% - 89.2%). The false-negative rate for ycN0 clipped nodes was 20%, whereas for non-clipped nodes, it was 11% (2).
Table 2 - Pre-NAC Axillary Ultrasound Performance for Malignant Lymph Node Detection
Comparison with Existing Literature
Our results are consistent with findings from the ACOSOG Z1071 trial, which reported an SLNB false-negative rate of 12.6%, comparable to our observed rate of 11-20% (18). The SENTINA trial reported lower SLN detection rates post-NAC, which aligns with our findings demonstrating the limited sensitivity of post-NAC US (28.6%) (19). The SN FNAC study showed an SLNB accuracy post-NAC of 92.3%, slightly higher than our observed accuracy of 82.5% (20). A direct comparison with published trials is shown in table 5.
Table 3 - Post-NAC Axillary Ultrasound Performance for Malignant Lymph Node Detection

Table 4 - Histopathological and Clinical Features of Breast Cancer Cases
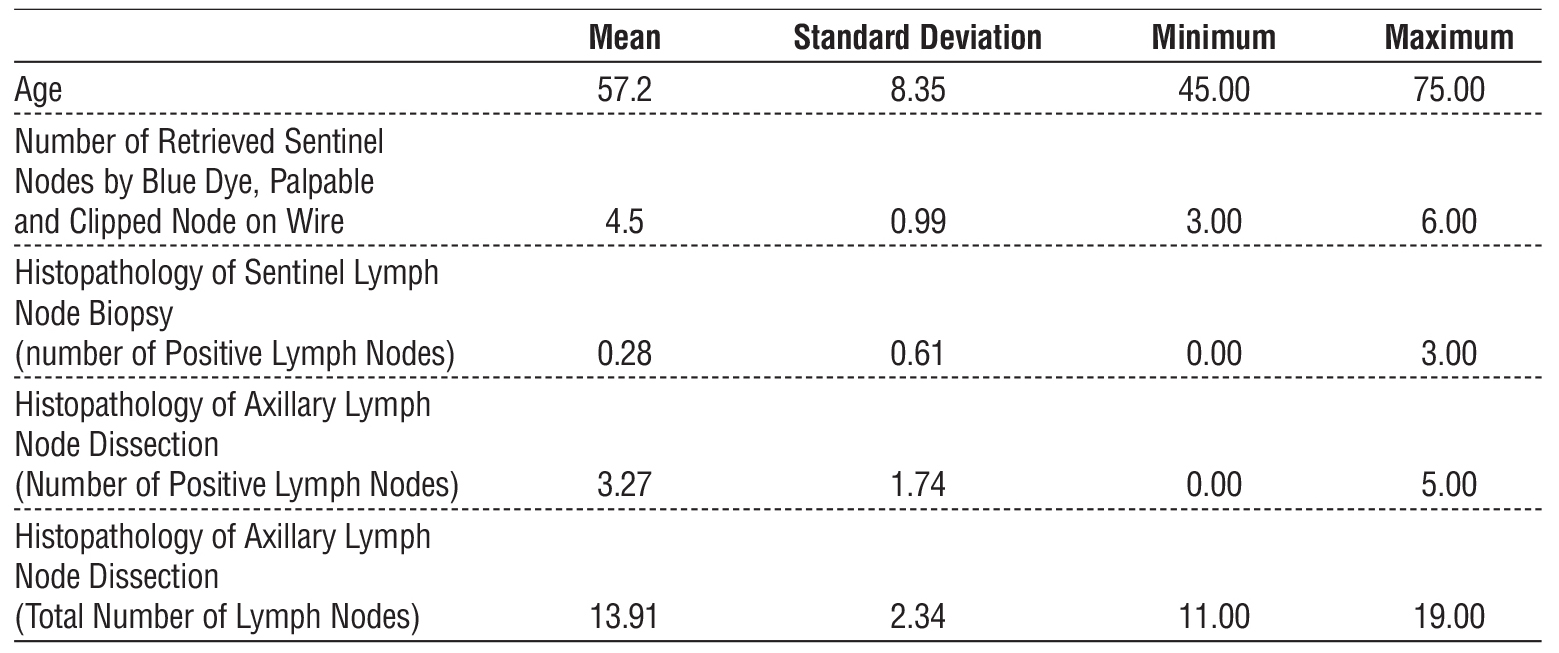
Table 5 - Comparison of false-negative rates (FNR) with existing literature (ACOSOG Z1071, SENTINA, SN FNAC)
Summary of Key Findings
Post-NAC axillary US had high specificity (94%) but low sensitivity (28.6%), limiting its role as a standalone diagnostic tool. The high conversion rate to ycN0 (90%) supports the feasibility of axillary de-escalation strategies. Clipped node localization improved nodal assessment, reducing the SLNB false-negative rate compared to historical studies. However, histopathological confirmation remains essential, as US alone cannot reliably rule out residual nodal disease.
DISCUSSION
This study evaluated the diagnostic performance of axillary ultrasound (US) in assessing nodal response to neoadjuvant chemotherapy (NAC) in early-stage breast cancer. The findings indicate that post-NAC US has a high specificity (94%) and negative predictive value (86.1%), making it a useful tool in identifying patients who may avoid unnecessary axillary lymph node dissection (ALND). However, its sensitivity remains low (28.6%), emphasizing the continued need for histopathological confirmation.
Comparison with Existing Literature
The results align with prior studies that report high specificity but limited sensitivity of axillary US post-NAC. The ACOSOG Z1071 trial found an SLNB false-negative rate (FNR) of 12.6% when conducted post-NAC, which is comparable to our observed FNR of 11-20% (21). Similarly, the SENTINA trial demonstrated lower SLN detection rates after NAC, reinforcing the finding that post-NAC US alone is not a reliable predictor of residual nodal disease (22). The SN FNAC study reported an SLNB accuracy of 92.3%, which is slightly higher than our observed accuracy of 82.5% (23).
Despite these findings, the variability in FNR among different studies underscores the influence of institutional protocols, imaging techniques, and operator expertise. The discrepancy in SLNB accuracy highlights the need for standardization in axillary US interpretation and integration with other diagnostic modalities, such as MRI or AI-based image processing, to enhance reliability.
Clinical Implications
The high conversion rate to ycN0 (90%) in this study supports the growing trend toward de-escalating axillary surgery in patients achieving nodal clearance post-NAC. Patients with ycN0 status on post-NAC US may be candidates for sentinel lymph node biopsy (SLNB) rather than ALND, potentially reducing morbidity without compromising oncologic safety. However, given the limited sensitivity of US, histopathological confirmation remains essential to prevent undertreatment of residual nodal disease. Histopatho-logical outcomes of SLNB and ALND stratified by ycN status are detailed in table 6.
Table 6 - Histopathological outcomes of SLNB and ALND
In clinical practice, these findings suggest that while axillary US is valuable in guiding surgical decision-making, it cannot replace SLNB. The integration of axillary US with other imaging modalities may further refine preoperative staging, optimizing treatment plans for patients undergoing NAC.
Limitations
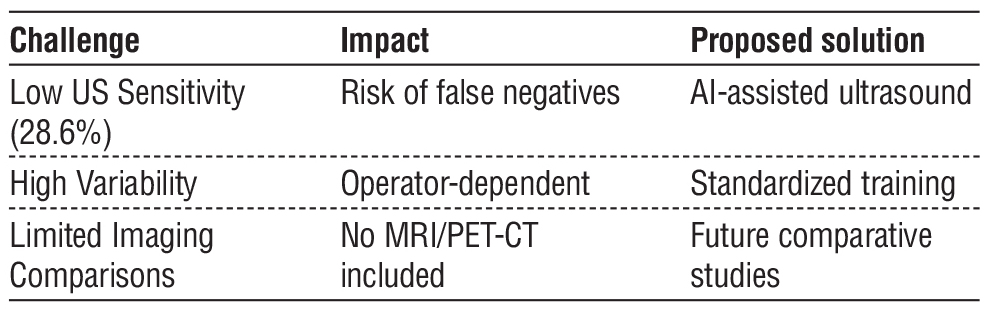
Despite the promising findings, this study has several limitations. First, it was conducted at a single center with a modest sample size, which may limit generalizability. Second, the lack of long-term follow-up prevents assessment of recurrence rates and overall survival. Additionally, inter-reader variability in ultrasound interpretation was not analyzed, which may affect diagnostic consistency.
Furthermore, the absence of a direct comparison with other imaging modalities, such as MRI or PET-CT, limits the ability to contextualize the performance of axillary US within a broader diagnostic framework. Future research should address these gaps to refine the role of axillary US in post-NAC evaluation.
Recommendations:
· Extensive multicenter studies with larger sample sizes are required to enhance the robustness of the findings.
· More in-depth statistical analysis is needed to validate the results.
· Additional research is necessary to assess the role of ultrasound in advanced breast cancer.
Future Directions
Further multicenter studies with larger cohorts are needed to validate these findings. Incorporating artificial intelligence (AI) into US image analysis may improve diagnostic accuracy and reduce variability. Additionally, longer follow-up studies are required to determine the impact of post-NAC US assessment on long-term oncologic outcomes. Comparative studies evaluating US alongside MRI or PET-CT could provide a more comprehensive understanding of its utility in post-NAC staging. Advancements in real-time elasto-graphy and contrast-enhanced ultrasound may also enhance axillary US accuracy, reducing reliance on invasive procedures. Future studies should explore these techniques to improve nodal assessment and guide personalized surgical management in breast cancer patients.
CONCLUSION
This study highlights the role of axillary ultrasound (US) as a valuable tool in evaluating nodal response to neoadjuvant chemotherapy (NAC) in early-stage breast cancer. With its high specificity and negative predictive value, axillary US may aid in identifying patients who can safely undergo less invasive surgical approaches such as sentinel lymph node biopsy (SLNB) rather than axillary lymph node dissection (ALND). However, its limited sensitivity underscores the need for histopathological confirmation to prevent under-treatment of residual nodal disease.
Future research should focus on refining imaging techniques, integrating artificial intelligence (AI), and conducting long-term follow-up studies to assess oncologic outcomes. A multimodal approach that combines axillary US with additional imaging techniques like MRI or PET-CT could enhance diagnostic accuracy and guide individualized surgical decision-making (table 7).
Table 7 - Proposed future advancements in imaging
Highlights
· Post-NAC axillary US demonstrates high specificity (94%) but low sensitivity (28.6%).
· 90% of patients converted to ycN0, supporting surgical de-escalation.
· SLNB accuracy post-NAC was 82.5%, compared to 92.3% in SN FNAC trial.
· Histopathological confirmation remains essential to minimize false negatives.
· Future studies should integrate AI and multimodal imaging for improved accuracy.
Ethical Consideration
1. Informed Consent: Before participation, each patient provided informed consent after receiving a clear explanation of the study's purpose, scope, and potential implications in a comprehensible manner.
2. Confidentiality: Patient confidentiality was strictly upheld. Only initials were recorded in the case report form, while any documents containing full names were securely stored by the investigators. A confidential patient identification list (linking initials to full names) was maintained to ensure accurate record identification.
3. Protocol Approval: The study protocol and all associated documents were reviewed and approved in compliance with local regulations. Ethical and research approval was granted by the Council of the General Surgery Department, Cairo University, under ethical committee approval number MD_72_2023.
4. Safety and Efficacy: No harmful effects related to the study interventions were observed.
Conflict of Interest Statement
The authors declare no conflicts of interest related to this study.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical Approval
This study was approved by the Ethics Committee of the Faculty of Medicine, Cairo University (approval number MD_72_2023). Written informed consent was obtained from all participants prior to enrollment. The study was conducted in accordance with the ethical standards of the Declaration of Helsinki.
REFERENCES
1. Banys-Paluchowski M, Gruber IV, Hartkopf A, Paluchowski P, Krawczyk N, Marx M, et al. Axillary ultrasound for prediction of response to neoadjuvant therapy in the context of surgical strategies to axillary dissection in primary breast cancer: a systematic review of the current literature. Arch Gynecol Obstet. 2020;301(2):341-53.
2. Riedel F, Schaefgen B, Sinn HP, Feisst M, Hennigs A, Hug S, et al. Diagnostic accuracy of axillary staging by ultrasound in early breast cancer patients. Eur J Radiol. 2021;135:109468.
3. Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310(14):1455-61.
4. Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14(7):609-18.
5. Caudle AS, Yang WT, Krishnamurthy S, Mittendorf EA, Black DM, Gilcrease MZ, et al. Improved Axillary Evaluation Following Neoadjuvant Therapy for Patients With Node-Positive Breast Cancer Using Selective Evaluation of Clipped Nodes: Implementation of Targeted Axillary Dissection. J Clin Oncol. 2016;34(10):1072-8.
6. Maeshima Y, Sakai T, Ogiya A, Takahashi Y, Miyagi Y, Kokubu Y, et al. Assessment of axillary node status by ultrasound after neoadjuvant chemotherapy in patients with clinically node-positive breast cancer according to breast cancer subtype. Sci Rep. 2021;11(1):10858.
7. Kim HS, Shin MS, Kim CJ, Yoo SH, Yoo TK, Eom YH, et al. Improved Model for Predicting Axillary Response to Neoadjuvant Chemotherapy in Patients with Clinically Node-Positive Breast Cancer. J Breast Cancer. 2017;20(4):378-85.
8. Cao S, Liu X, Cui J, Liu X, Zhong J, Yang Z, et al. Feasibility and reliability of sentinel lymph node biopsy after neoadjuvant chemotherapy in breast cancer patients with positive axillary nodes at initial diagnosis: An up-to-date meta-analysis of 3,578 patients. Breast. 2021;59:256-69.
9. Kühn T, Classe JM, Gentilini OD, Tinterri C, Peintinger F, de Boniface J. Current Status and Future Perspectives of Axillary Management in the Neoadjuvant Setting. Breast Care (Basel). 2018;13(5):337-41.
10. Giuliano AE, Ballman KV, McCall L, et al. Effect of Axillary Dissection vs No Axillary Dissection on 10-Year Overall Survival Among Women With Invasive Breast Cancer and Sentinel Node Metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA. 2017;318(10):918-926.
11. Hanna Piotrzkowska-Wróblewska, Katarzyna Dobruch-Sobczak, Ziemowit Klimonda, et al. Monitoring breast cancer response to neoadjuvant chemotherapy with ultrasound signal statistics and integrated backscatter. arXiv preprint arXiv:1901.03818. 2019.
12. Jagsi R, Chadha M, Moni J, et al. Radiation Field Design in the ACOSOG Z0011 (Alliance) Trial. J Clin Oncol. 2014;32(32):3600-3606.
13. Giuliano AE, Hunt KK, Ballman KV, et al. Axillary Dissection vs No Axillary Dissection in Women With Invasive Breast Cancer and Sentinel Node Metastasis: A Randomized Clinical Trial. JAMA. 2011;305(6):569-575.
14. Giuliano AE, Ballman KV, McCall L, et al. Locoregional Recurrence After Sentinel Lymph Node Dissection With or Without Axillary Dissection in Patients With Sentinel Lymph Node Metastases: Long-term Follow-Up From the American College of Surgeons Oncology Group (Alliance) ACOSOG Z0011 Randomized Trial. Ann Surg. 2016;264(3):413-420.
15. Boileau JF, Poirier B, Basik M, Holloway CM, Gaboury L, Sideris L, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015;33(3):258-64.
16. Annals of Surgical Oncology. Clip Placement in Positive Lymph Nodes Before Neoadjuvant Chemotherapy and Its Impact on Axillary Surgery. Ann Surg Oncol. 2024
17. Cancers. Comprehensive Axillary Management of Clinically Node-Positive Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy. Cancers (Basel). 2024;16(19):3354.
18. JAMA Surgery. Safety of Targeted Axillary Dissection After Neoadjuvant Therapy in Clinically Node-Positive Breast Cancer Patients. JAMA Surg. 2023.
19. World Journal of Surgical Oncology. Axillary Management and Long-Term Oncologic Outcomes in Breast Cancer. World J Surg Oncol. 2024;22:25.
20. Cancers. Axillary Surgery for Breast Cancer in 2024. Cancers (Basel). 2024;16(9):1623.
21. European Journal of Surgical Oncology. Current Axillary Management of Patients with Early Breast Cancer in the UK: A National Survey. Eur J Surg Oncol. 2023.
22. El-Tamer M, Kovacs T. Management of the axilla in T1-2N1 breast cancer. npj Breast Cancer. 2022;8(1):69.
Full Text Sources:
Abstract:
Views: 22

For Authors
Journal Subscriptions

Sept 2025
Supplements
Instructions for authors
Online submission
Contact
e-ISSN: 2601 - 1700 (online)
ISSN-L: 2559 - 723X
Journal Abbreviation: Surg. Gastroenterol. Oncol.
Surgery, Gastroenterology and Oncology (SGO) is indexed in:
- SCOPUS
- EBSCO
- DOI/Crossref
- Google Scholar
- SCImago
- Harvard Library
- Open Academic Journals Index (OAJI)
Surgery, Gastroenterology and Oncology (SGO) is an open-access, peer-reviewed online journal published by Celsius Publishing House. The journal allows readers to read, download, copy, distribute, print, search, or link to the full text of its articles.
Time to first editorial decision: 25 days
Rejection rate: 61%
CiteScore: 0.2

Meetings and Courses in 2025
Meetings and Courses in 2024
Meetings and Courses in 2023
Meetings and Courses in 2022
Meetings and Courses in 2021
Meetings and Courses in 2020
Meetings and Courses in 2019
Verona expert meeting 2019

Surgery, Gastroenterology and Oncology applies the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits readers to copy and redistribute the material in any medium or format, remix, adapt, build upon the published works non-commercially, and license the derivative works on different terms, provided the original material is properly cited and the use is non-commercial. Please see: https://creativecommons.org/licenses/by-nc/4.0/
Publisher’s Note:
The opinions, statements, and data contained in article are solely those of the authors and not of Surgery, Gastroenterology and Oncology journal or the editors. Publisher and the editors disclaim responsibility for any damage resulting from any ideas, instructions, methods, or products referred to in the content.
 IASGO Society News
IASGO Society News