Surgery, Gastroenterology and Oncology
|
|
Introduction
Cervical cancer ranks as the second leading malignancy among women, particularly in developing regions where organized screening and HPV vaccination programs remain limited. Despite advances in prevention, the disease continues to represent a serious public health challenge. Globally, the incidence rate is estimated at 13.3 per 100,000 women-years, with a mortality rate of approximately 7.2 per 100,000 women-years (1).
Persistent infection with high-risk HPV types is the main causal factor in cervical carcinogenesis. Following epithelial infection, HPV integrates into host DNA, leading to deregulated expression of viral oncogenes E6 and E7, which inactivate p53 and retinoblastoma (Rb) tumor suppressor pathways. These molecular events drive the transformation from cervical intraepithelial neoplasia (CIN) to invasive carcinoma (2,3).
Most infections are transient and cleared within a year due to effective innate and adaptive immune responses. However, when immune surveillance fails - whether due to viral, environmental, or host factors -persistent HPV infection may evolve into precancerous or cancerous lesions (4).
Regulatory T cells (Tregs), characterized by FOXP3 expression, have been recognized as crucial modulators of this process. While Tregs maintain immune tolerance, their overactivation may suppress cytotoxic lymphocyte function and facilitate immune evasion by tumor cells (5,6). The following sections outline FOXP3’s function in immune regulation and its role in cervical cancer progression (fig. 1).
Figure 1 - Overview of HPV-induced cervical carcinogenesis and FOXP3 involvement
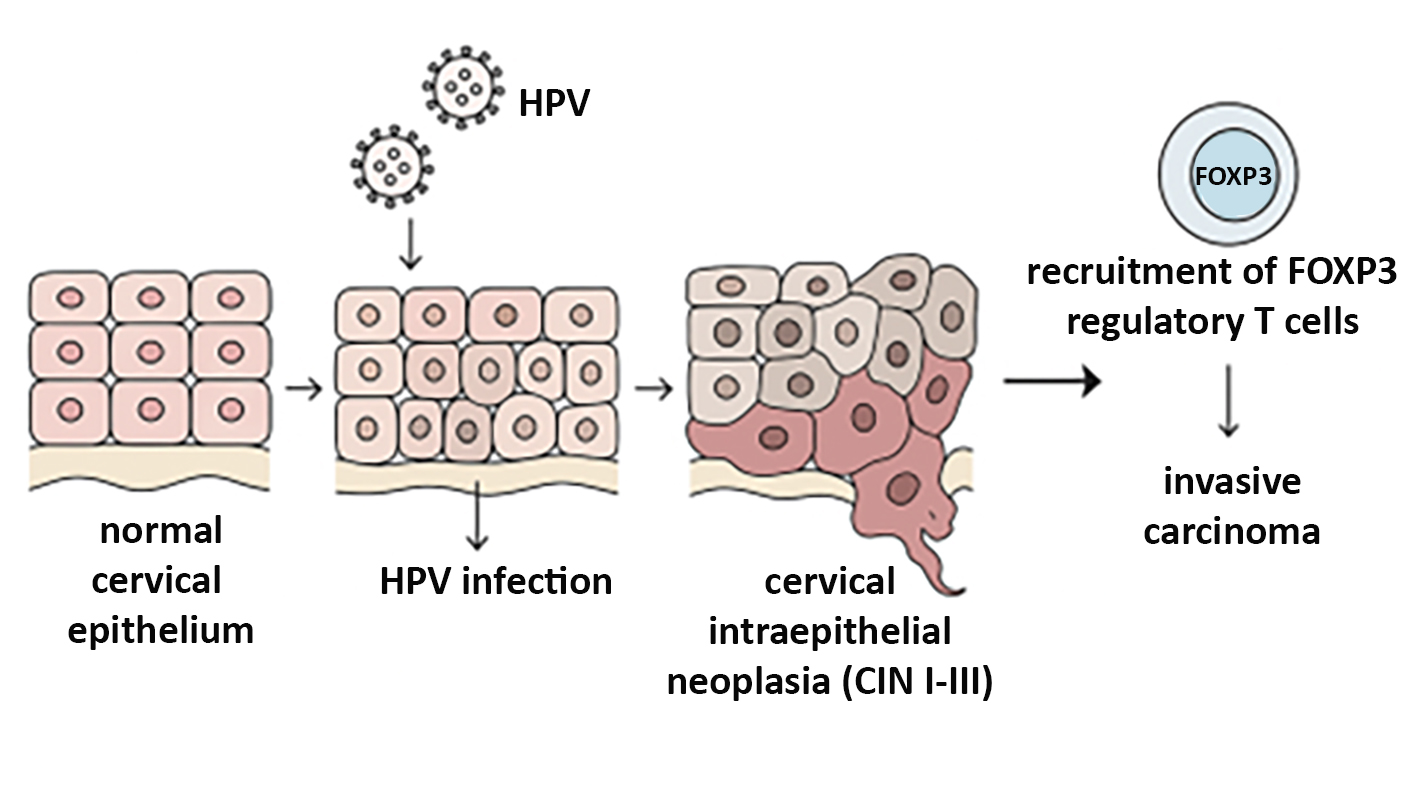
Schematic showing the sequential transformation from normal cervical epithelium to HPV infection, CIN I–III, and invasive carcinoma. Viral genome integration, abnormal epithelial proliferation, and recruitment of FOXP3+ Tregs contribute to immune suppression and tumor progression (made with artificial intelligence).
Foxp3 and Immune Regulation
The immune system balances activation against pathogens with mechanisms that prevent self-damage. Regulatory T cells (Tregs) are central to this equilibrium. They suppress exaggerated immune responses and maintain peripheral tolerance through cytokines such as IL-10 and TGF-?, expression of inhibitory receptors (CTLA-4, PD-1), and modulation of antigen-presenting cells (7,8).
FOXP3, a forkhead transcription factor, orchestrates the development and function of Tregs by regulating genes responsible for their suppressive phenotype (9). Sustained FOXP3 expression is required to preserve Treg stability and metabolic fitness.
In the tumor microenvironment (TME), this regulatory role becomes a double-edged sword. While FOXP3? Tregs help prevent chronic inflammation, they can also inhibit anti-tumor cytotoxic T lymphocytes (CTLs) and natural killer (NK) cell activity, promoting immune tolerance to tumor antigens (10,11).
The cervical cancer microenvironment is a complex interplay of epithelial, stromal, and immune cells embedded within a cytokine-rich extracellular matrix. Treg accumulation in this milieu correlates with tumor growth, angiogenesis, and metastasis, underscoring FOXP3’s relevance as an immune suppressor in gynecologic oncology (12).
Foxp3 and Cervical Cancer
Persistent infection with oncogenic HPV types induces dysplastic changes within the cervical epithelium - progressing through CIN1 (mild), CIN2 (moderate), and CIN3 (severe) stages before the onset of invasive carcinoma (13).
Numerous studies demonstrate that FOXP3 expression increases in both tumor-infiltrating Tregs and cancer cells themselves during cervical cancer progression (14-17). Overexpression of FOXP3 correlates with enhanced tumor proliferation, invasiveness, and poor prognosis. Experimental data show that FOXP3 can alter the cell cycle, suppress apoptosis, and promote lymphangiogenesis within cervical cancer tissue (16,17).
Increased density of FOXP3? Tregs in the tumor stroma is associated with reduced CTL and NK-cell activity, leading to immune escape (fig. 2). This relationship highlights FOXP3’s dual function: maintaining immune homeostasis but simultaneously shielding tumor cells from immune attack.
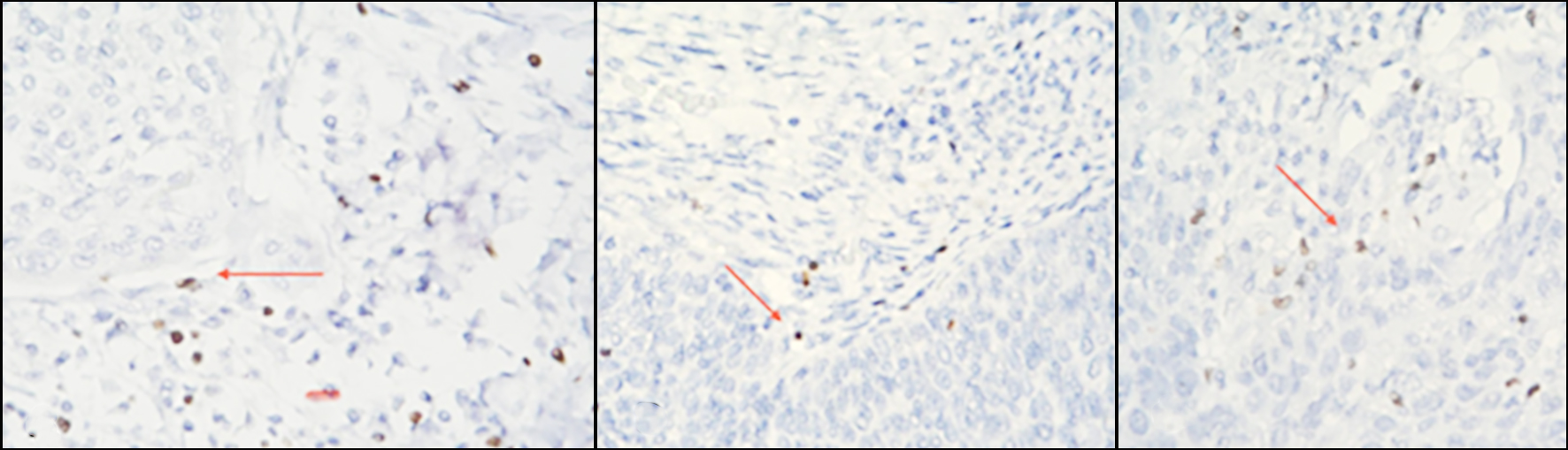
Figure 2 - Microscopic evaluation of IHC- stained FOXP3+ T cells (colored brownish) in CC: absent (a); low (b) and high (c) intratumoral infiltration of positive cells (red arrow).
FOXP3 as a Prognostic Biomarker
The identification of reliable biomarkers for early cervical cancer detection remains a major research goal. FOXP3 has emerged as a potential immunohistochemical and molecular marker due to its consistent association with tumor aggressiveness and patient outcome (18,19).
In normal cervical epithelium, FOXP3? cells are scarce. Their number increases progressively through CIN stages and peaks in invasive lesions. Elevated FOXP3 levels have been linked to reduced survival rates and higher recurrence risk, making it a negative prognostic indicator in most cervical and ovarian cancers.
Interestingly, FOXP3’s prognostic value appears tissue-specific. In colorectal and some breast cancers, high FOXP3 expression correlates with improved prognosis, emphasizing the context-dependent nature of its function. In HPV-related malignancies, however, FOXP3 is primarily associated with immune tolerance and unfavorable outcomes.
The dynamic distribution of tumor-infiltrating lymphocytes (TILs) -including CD3?, CD4?, CD8?, and FOXP3? populations -provides a valuable immunological landscape for evaluating disease progression and therapeutic response (20) (fig. 3).
Figure 3 - Foxp3 expression in cervical cancer
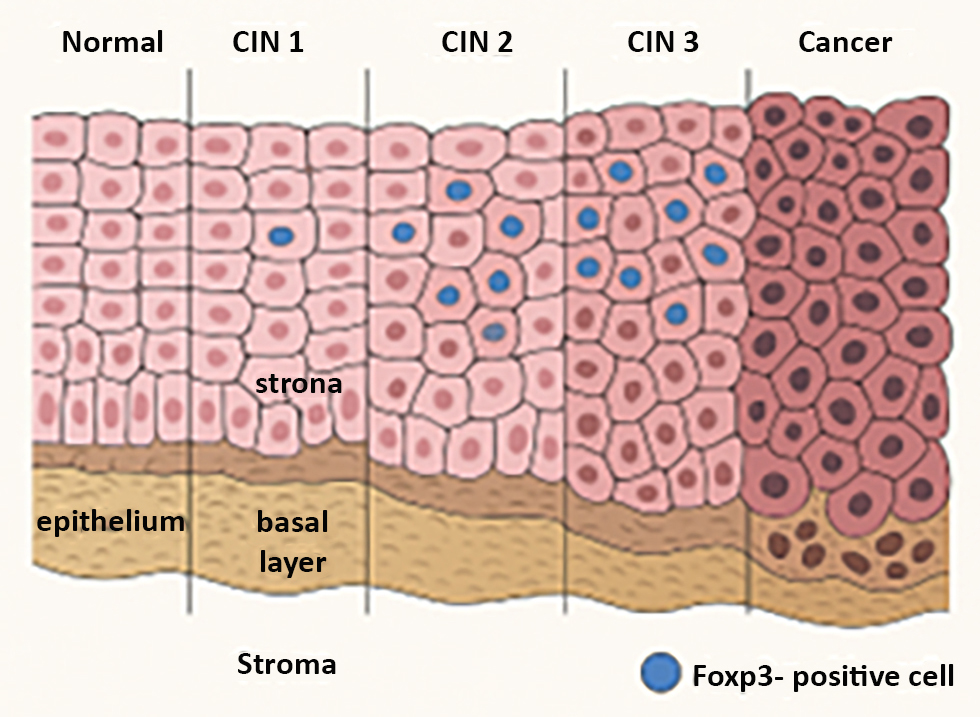
(made with artificial intelligence)
Targeting FOXP3 and Tregs in Cervical Cancer Therapy
Given the central role of FOXP3? Tregs in immunosuppression, therapeutic strategies aimed at modulating or depleting these cells are of growing interest. Selective targeting of tumor-infiltrating Tregs — while sparing peripheral populations necessary for self-tolerance — represents a promising approach (21,22).
One potential method involves silencing FOXP3 expression through RNA interference or small-molecule inhibitors, thereby reducing Treg suppressive capacity and restoring effector T-cell activity (23). Another avenue is the development of vaccines that elicit immune responses against FOXP3, which have shown efficacy in preclinical models by preferentially depleting intratumoral Tregs without inducing systemic auto-immunity (24,25).
Moreover, combining Treg-modulating agents with immune checkpoint inhibitors (e.g., anti-PD-1, anti-CTLA-4 antibodies) could synergistically enhance antitumor responses. (23). Metabolic reprogramming of Tregs, particularly targeting their dependence on oxidative phosphorylation, has also emerged as an innovative strategy to suppress their function within the tumor microenvironment without systemic toxicity.
Given the central role of FOXP3? Tregs in immunosuppression, therapeutic strategies aimed at modulating or depleting these cells are of growing interest. Selective targeting of tumor-infiltrating Tregs — while sparing peripheral populations necessary for self-tolerance — represents a promising approach (21,22).
One potential method involves silencing FOXP3 expression through RNA interference or small-molecule inhibitors, thereby reducing Treg suppressive capacity and restoring effector T-cell activity (23). Another avenue is the development of vaccines that elicit immune responses against FOXP3, which have shown efficacy in preclinical models by preferentially depleting intratumoral Tregs without inducing systemic auto-immunity (24,25).
Moreover, combining Treg-modulating agents with immune checkpoint inhibitors (e.g., anti-PD-1, anti-CTLA-4 antibodies) could synergistically enhance antitumor responses. (23). Metabolic reprogramming of Tregs, particularly targeting their dependence on oxidative phosphorylation, has also emerged as an innovative strategy to suppress their function within the tumor microenvironment without systemic toxicity.
CONCLUSION
FOXP3 is a master regulator of immune tolerance and a central player in cervical cancer immunobiology. Its overexpression fosters an immunosuppressive microenvironment conducive to tumor persistence and progression. As both a biomarker and a therapeutic target, FOXP3 holds significant promise for improving diagnosis and treatment personalization in cervical cancer.
Future research should clarify the molecular mechanisms regulating FOXP3 expression in HPV-related malignancies, explore differential roles across HPV subtypes, and develop clinically feasible methods for modulating FOXP3 activity. Integration of FOXP3 profiling into individualized therapeutic strategies - particularly in combination with immunotherapy or HPV-directed vaccines - may substantially improve patient outcomes.
Author’s Contribution
All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Competing Interests
Authors state no conflict of interest.
Research Funding: None declared.
Data Availability
The authors declare that all related data are available concerning researchers by the corresponding author’s email.
REFERENCES
1. Singh D, Vignat J, Lorenzoni V, Eslahi M, Ginsburg O, Lauby- Secretan B, et al. Global estimates of incidence and mortality of
cervical cancer in 2020: a baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob Health. 2023; 11(2):e197-e206.
2. zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2(5):342-50.
3. Nakamura M, Obata T, Daikoku T, Fujiwara H. 2019. The Association and Significance of p53 in Gynecologic Cancers: The Potential of Targeted Therapy. Int J Mol Sci. 2019;20(21):5482.
4. Luo Q, Zhang S, Wei H, Pang X, Zhang H. Roles of Foxp3 in the occurrence and development of cervical cancer. Int J Clin Exp Pathol. 2015;8(8):8717-30.
5. Vignali DAA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8(7):523-32.
6. Maxwell Parkin D, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006;24 Suppl 3:S3/11-25.
7. Bacchetta R, Gambineri E, Roncarolo M. Role of regulatory T cells and FOXP3 in human diseases. J Allergy Clin Immunol. 2007; 120(2):227-35; quiz 236-7.
8. Tan G, Wang Y, Zheng Y. The roles of regulatory T cells in cancer immunity and immunotherapy. J Clin Invest Volume 121(3):1034- 1041.
9. Georgiev P, Charbonnier L, Chatila TA. Regulatory T Cells: the Many Faces of Foxp3. J Clin Immunol. 2019;39(7):623-640.
10. Sakaguchi S, Vignali DAA, Rudensky AY, Niec RE, Waldmann H. The plasticity and stability of regulatory T cells. Nat Rev Immunol. 2013; 13(6):461-7
11. Chaudhary B, Elkord E. Regulatory T Cells in the Tumor Microenvironment and Cancer Progression: Role and Therapeutic Targeting. Vaccines (Basel). 2016;4(3):28.
12. Bozyk A, Wojas-Krawczyk K, Krawczyk P, Milanowski J. Tumor Microenvironment-A Short Review of Cellular and Interaction Diversity. Biology (Basel). 2022;11(6):929.
13. Snijders PJ, Steenbergen RD, Heideman DA, Meijer CJ. HPV - mediated cervical carcinogenesis: Concepts and clinical implications. J Pathol. 2006;208(2):152-64..
14. Kim JH, Kim BS, Lee SK. Regulatory T Cells in Tumor Micro-environment and Approach for Anticancer Immunotherapy. Immune Netw. 2020;20(1):e4.
15. Li L, Xu XT, Wang LL, Qin SB, Zhou JY. Expression and clinico-patho- logical significance of Foxp3 and VISTA in cervical cancer. Am J Transl Res. 2021;13(9):10428-10438.
16. Liu Y, Tu H, Zhang L, Xiong J, Li L. FOXP3-induced LINC00885 promotes the proliferation and invasion of cervical cancer cells. Mol Med Rep. 2021;23(6):458.
17. Huang C, Zhou L, Chang X, Pang X, Zhang H, Zhang S. B7-H3, B7- H4, Foxp3 and IL-2 expression in cervical cancer: Associations with patient outcome and clinical significance. Oncol Rep. 2016;35(4): 2183-90.
18. Volkova LV, Pashov AI, Omelchuk NN. Cervical Carcinoma: Oncobiology and Biomarkers. Int J Mol Sci. 2021;22(22):12571.
19. Wagner PD, Srivastava S. New paradigms in translational science research in cancer biomarkers. Transl Res. 2012;159(4):343-53.
20. Fleischmann M, Chatzikonstantinou G, Fokas E, Wichmann J, Christiansen H, Strebhardt K, et al. Molecular Markers to Predict Prognosis and Treatment Response in Uterine Cervical Cancer. Cancers (Basel). 2021;13(22):5748.
21. Frahm M, Bachmann M, Wild K. Targeting regulatory T cells in cancer immunotherapy. Nature Reviews Cancer. 2011;11(12):810-818.
22. Tanaka A, Sakaguchi S. Regulatory T cells in cancer immuno-therapy. Cell Res. 2017;27(1):109-118.
23. Chandran S, Wang H, Nair N. Targeting Tregs to enhance cancer immunotherapy. Cancer Research. 2020;80(3):536–543.
24. Mousavi-Niri N, Naseroleslami M, Hadjati J. Anti-regulatory T cell vaccines in immunotherapy: focusing on FoxP3 as target. Hum Vaccin Immunother. 2019;15(3):620-624.
25. Nair S, Boczkowski D, Fassnacht M, Pisetsky D, Gilboa E. Vaccination against the Forkhead Family Transcription Factor Foxp3 Enhances Tumor Immunity. Cancer Res. 2007;67(1):371-80.
Full Text Sources:
Abstract:
Views: 10

For Authors
Journal Subscriptions

Sept 2025
Supplements
Instructions for authors
Online submission
Contact
e-ISSN: 2601 - 1700 (online)
ISSN-L: 2559 - 723X
Journal Abbreviation: Surg. Gastroenterol. Oncol.
Surgery, Gastroenterology and Oncology (SGO) is indexed in:
- SCOPUS
- EBSCO
- DOI/Crossref
- Google Scholar
- SCImago
- Harvard Library
- Open Academic Journals Index (OAJI)
Surgery, Gastroenterology and Oncology (SGO) is an open-access, peer-reviewed online journal published by Celsius Publishing House. The journal allows readers to read, download, copy, distribute, print, search, or link to the full text of its articles.
Time to first editorial decision: 25 days
Rejection rate: 61%
CiteScore: 0.2

Meetings and Courses in 2025
Meetings and Courses in 2024
Meetings and Courses in 2023
Meetings and Courses in 2022
Meetings and Courses in 2021
Meetings and Courses in 2020
Meetings and Courses in 2019
Verona expert meeting 2019

Surgery, Gastroenterology and Oncology applies the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits readers to copy and redistribute the material in any medium or format, remix, adapt, build upon the published works non-commercially, and license the derivative works on different terms, provided the original material is properly cited and the use is non-commercial. Please see: https://creativecommons.org/licenses/by-nc/4.0/
Publisher’s Note:
The opinions, statements, and data contained in article are solely those of the authors and not of Surgery, Gastroenterology and Oncology journal or the editors. Publisher and the editors disclaim responsibility for any damage resulting from any ideas, instructions, methods, or products referred to in the content.
 IASGO Society News
IASGO Society News