Surgery, Gastroenterology and Oncology

|
|
Background: Laparoscopic cholecystectomy (LC) is a common operation in abdominal surgery. Injury to bile duct during LC is still relatively high. The emerging fluorescence-based intraoperative near-infrared cholangiography by Indocyanine green (ICG) and intraoperative cholangiogram (IOC) are mainly two intraoperative imaging techniques to delineate extra-hepatic biliary tree during LC.
Material and Methods: This prospective non randomized clinical study included 50 consecutive patients underwent elective laparoscopic cholecystectomy with no history of acute attack nor obstructive jaundice. All patients had been subjected to two different cholangiography techniques in the same patient. First, preoperative intravenous injection of ICG, and secondly, conventional intraoperative cholangiogram for more delineation of extrahepatic biliary tree. All cholecystectomies were performed in Theodor Bilharz Research Institute (TBRI) hospital during the period from November 2021 to August 2022. Patients were analyzed pre-operative, intra-operative & post-operative.
Results: Females represented almost the included participants (80%), mean age was 40.5 and mean BMI was 31.92. The mean total operative time was 82.5 min. & mean IOC time was 26.14 min. Visualization of CD, CHJ, CBD and CHD were more obvious by using ICG intravenously than conventional visual inspection before dissection of Calot’s triangle with significant statistical difference (P value < 0.001). After dissection of Calot’s triangle, there is no statistical difference between using ICG and conventional IOC in visualization of extrahepatic biliary. No adverse reactions were recorded. No Complications were detected.
Conclusion: Our results suggest that IOC using ICG is a feasible, safe, fast and more effective technique than conventional IOC for biliary tract identification before and after dissection of Calot’s triangle and may constitute a powerful diagnostic test for delineation of extrahepatic biliary tract in laparoscopic cholecystectomy.
INTRODUCTION
Treatment for symptomatic gallstone disease is regarded as gold standard, with laparoscopic cholecystectomy. To carry out a treatment safely, surgeons need to be well-versed in the architecture of the biliary tract (1).
Cholecystectomy using laparoscopy is a common procedure. Morbidity and death during LC are linked to biliary injury (2).
One method for intraoperative biliary tree visualization is intraoperative X-ray cholangiography. Nonetheless, there is disagreement on the strength of intraoperative X-ray cholangiography in preventing biliary injury (3).
To improve LC safety, a plethora of intraoperative imaging methods and tools have been created. Two primary intraoperative imaging approaches are the classic intraoperative cholangiogram (IOC) and the newly developed fluorescence-based intraoperative near-infrared cholangiography (NIR-C) employing indocyanine green (ICG) (4).
A serious side effect of laparoscopic cholecystec-tomy is biliary injury. Since biliary injury is the most common surgical complication, intraoperative cholangiography using indocyanine green (ICG) may help prevent biliary injury by enhancing vision of the extrahepatic biliary tree throughout the procedure (5).
For effective intraoperative orientation of the extrahepatic bile ducts, near-infrared fluorescence (NIRF) cholangiography following an intravenous administration of indocyanine green (ICG) is a more straightforward method than traditional laparoscopic imaging (6).
Aim of this research was to assess safety, effectiveness, feasibility and accuracy of intravenous administration of indocyanine green versus conventional intraoperative cholangiography for the best delineation of extra-hepatic biliary ducts and proper identification of biliary tree for prevention of biliary injury in laparoscopic cholecystectomy.
MATERIAL AND METHODS
Fifty consecutive patients were incorporated in this prospective non randomized clinical study who underwent elective laparoscopic cholecystectomy operation. All patients had been subjected to two different cholangiography techniques in the same patient. All cholecystectomies were performed in TBRI hospital.
Inclusion criteria
All patients aged from 18 to 65 complaining of a chronic calcular cholecystitis that were indicated for elective laparoscopic cholecystectomy and fit for operation.
Exclusion criteria
Patients with acute calcular cholecystitis, obstructive jaundice or contraindication for laparoscopic surgery (for example patients had significant pulmonary or cardiac problems or severe renal insufficiency). Proven or suspected allergies to iodine, urografin or ICG. Pregnancy or lactation. Mentally disabled patients.
Ethical approval
The research procedure was authorized by the "Research Ethics Committee" in the TBRI (PT 533) and the School of Medicine at Cairo University (Code: MD-129-2020), and each participant was asked to sign an informed consent document.
Methods
The study included the patients who were admitted in the surgery department – TBRI hospital during the period from November 2021 to August 2022.
All patients signed an informed consent for operation and cholangiography.
Patients were subjected to two different cholangio-graphy techniques: the first technique: preoperative intravenous indocyanine green (ICG) injection one hour before surgery then using near-infrared fluorescence imaging during the operation. The Second Technique: was performed by cystic duct cannulation with a catheter using grasper and injection of the urografin dye. A mobile X-ray C-arm system is used, and the monochrome X-ray image is shown on a separate screen.
Standard laparoscopic cholecystectomy
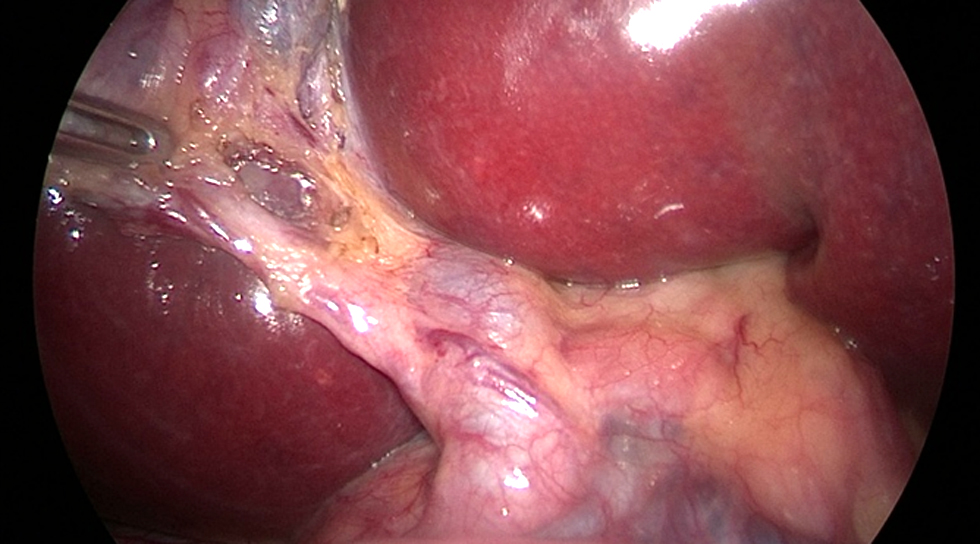
The supra-umbilical open procedure (Hasson's technique) was used to achieve pneumoperitoneum. LC made the four standard tiny incisions in the abdominal wall to introduce two 5 mm and two 10 mm diameter operating ports. Through those ports, the surgical instruments and telescope were inserted into the peritoneal cavity. In our study, the extrahepatic biliary tree was delineated using two cholangiography techniques: urografin and ICG. We'll go over both strategies in detail. The supra-umbilical port was observed with a 10 mm telescope. The surgeon used a second 10 mm port, his right working port, in the epigastric area. A 5 mm port in the right hypochondrial region served as the surgeon's left operating port, while another 5 mm port in the right lumbar region was employed for the traction of the gallbladder fundus. With the left hand, retracting Hartmann's pouch, while the right hand performed anterior and posterior dissection in Calot's triangle, creating a large window. A critical perspective on safety is crucial in preventing injuries to right hepatic artery or bile duct (fig. 1). To separate the gallbladder from its bed, the cystic duct and artery were cleaned, dissected, controlled with clips, and finally severed. The gallbladder was extracted through the epigastric port with closure of ports openings.
Figure 1 - Critical view of safety showing cystic duct, cystic artery & CBD.
Visualization of the gall bladder and bile ducts with Indocyanine Green (ICG) injection
One ICG vial 25 mg powder was diluted in 10 ml of sterile water so that 1 ml is equivalent to 2.5 mg and administered to patients one hour before surgery according to their body weight with a standard dose of 0.1 mg per Kg (2).
A laparoscopic imaging system (KARL STORZ GmbH & Co. KG, Tuttlingen, Germany) was utilized in each and every instance. The superior complete high-definition camera system (IMAGE 1 SPIESTM, KARL STORZ) produces the imaging. It is linked to a laparoscope with a 30° field of view and a 10 mm diameter that is fitted with a particular filter to enable the best possible detection of NIR fluorescence and white light without the need for manual switching.
The strong xenon light source (D-LIGHT P SCB, KARL STORZ) produces light that is both visible and near-infrared. The surgeon uses a pedal to control the switch from conventional light to NIR (7).
A professional image enhancement system (IMAGE 1 SPIESTM system, KARL STORZ GmbH & Co. KG, Tuttlingen, Germany) that enables customizable visualization modalities that can be chosen in accordance with surgeon preferences (7) improves visualization in both standard and NIR light. A critical understanding of safety is attained by carefully dissecting the cystic duct and clearly defining the junction between it and the CBD using NIR fluorescence light switching in conjunction with white light (figs. 2, 3).

The cystic duct and artery were identified and dissected, then controlled with clips and cut in order to remove the gallbladder from its bed. The gallbladder was removed through the epigastric port. Closures of ports openings were done.
Visualization of the bile tree using conventional urografin cholangiography (Figs. 4-9)
Cystic duct distal clipping is done to stop bile from the gallbladder from flowing backward. Just below the distal clip, the cystic duct's lumen circumference is cut in half. The cholangiogram catheter was inserted into the cystic duct via the right hypochondrial port. The cystic duct was then flushed with saline, and a water-soluble, diluted contrast material called "Urografin 76%" was injected into the catheter's free end. This material was free of air bubbles and contained 10 milliliters of urografin diluted with 10 milliliters of normal saline in a 20 milliliter syringe. The X-ray C-arm system was then used to radiographically follow the contrast wave. The cystic duct should be cut and double clipped near the entrance.

Throughout the entire procedure, the operating time was documented. Assessment of the length of time required for a conventional cholangiography technique, taking into account the fact that ICG was injected prior to the procedure, the extrahepatic biliary system's delineation, the identification of any CBD stones, the failure of the IOC, and any intraoperative complications. An assessment of the difficulties and hospital stay following surgery. The outcomes were noted, totaled, and subjected to statistical analysis.
Statistical analysis
To be statistically examined, pre-coded data was input into the computer using the statistical package of social science software, version 23 (SPSS). For quantitative variables, mean and standard deviation will be used to describe the data; for qualitative variables, number and percent will be used. Data normality is checked using the Shapiro-test of normality. When measuring a qualitative variable more than twice, the Cochrane tests compare the data over time. A pairwise comparison was done to determine how significant each pair was. Kappa measure used for agreement of qualitative variables. Cohen recommend the Kappa result be presumed as follows: values ≤ 0 as denoting 0.01–0.20 and no agreement as slight to none, 0.81–1.00 as almost perfect agreement, 0.61–0.80 as substantial, 0.41– 0.60 as moderate, 0.21–0.40 as fair, and P value is considered significant when equal to or less than 0.05.
RESULTS
Table 1 - Age, sex and BMI distribution of studied group
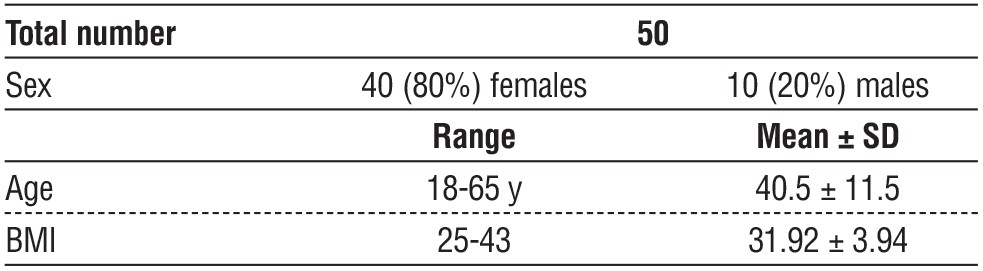
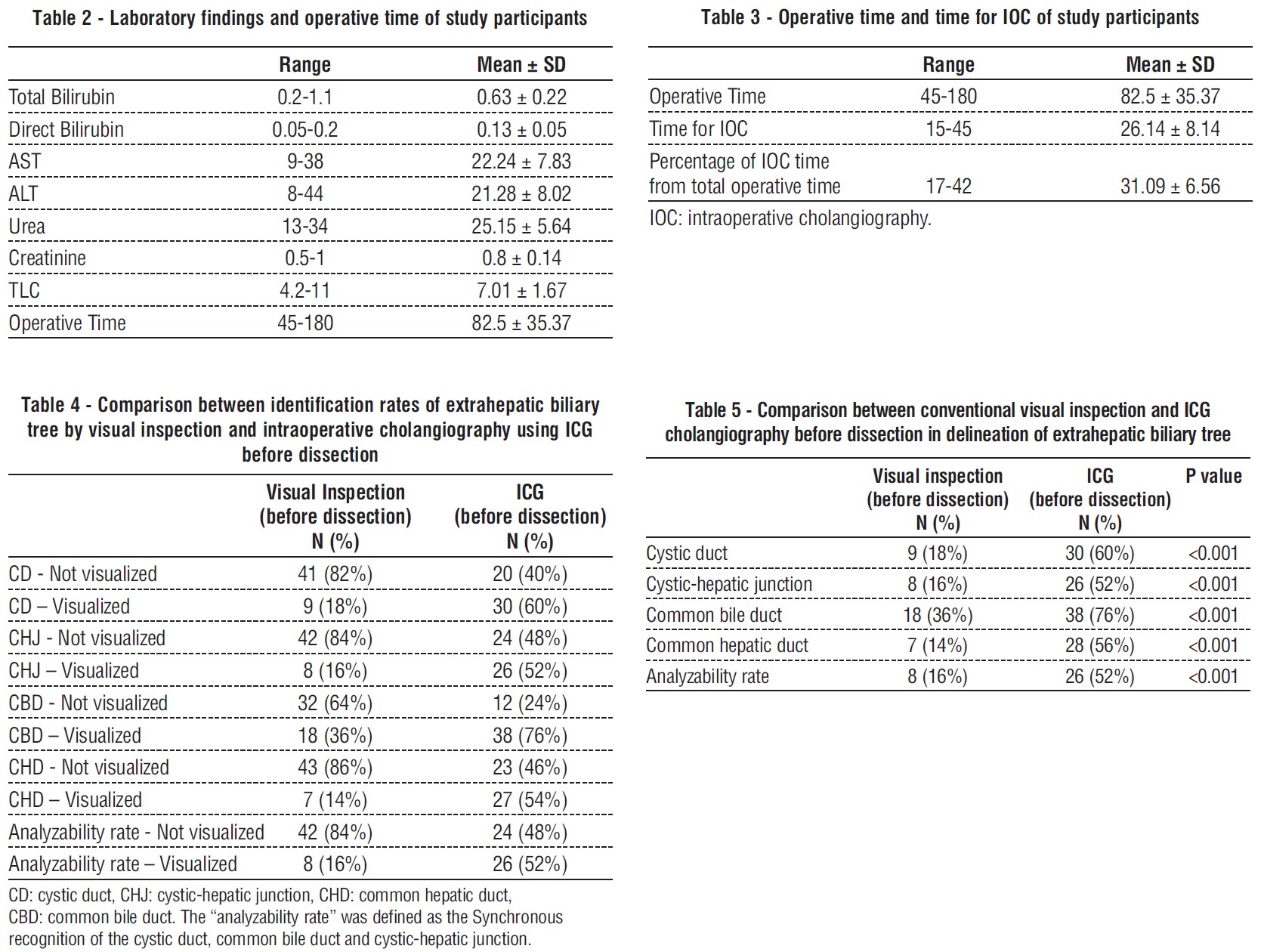
Figure 10 - Visual inspection versus ICG before dissection

Figure 11 - ICG versus conventional ICG after dissection

Table 6 - Comparison between identification rates of extrahepatic biliary tree by intraoperative cholangiography using ICG and conventional IOC after dissection

DISCUSSION
Conventional X-ray cholangiography is the almost used method for intraoperative visualization of the extrahepatic biliary system. Its regular application has been talked about since before laparoscopic cholecystectomies were invented. One benefit of this approach is that gallstones within the biliary channels can be seen intraoperatively. The requirement to cannulate and dissect the cystic duct before obtaining the initial cholangiogram is a drawback. Additionally, there is a chance that the treatment would damage the bile duct, which could lead to serious consequences. Its use is also restricted in the event that the cystic duct becomes blocked (8).
Table 7 - Comparison between ICG cholangiography and conventional IOC after dissection in delineation of extrahepatic biliary tree

Table 8 - Comparison between ICG cholangiography before and after dissection in delineation of extrahepatic biliary tree
No complications were detected neither intraoperative (bleeding or iatrogenic biliary injury) nor post-operative (bleeding, leakage, missed stone or wound infection) and no conversion to laparotomy. The hospital stay was one day and the patient discharged to home with follow up for one month.
Theoretically, intraoperative cholangiography with indocyanine green (IOC-ICG) is more successful than standard IOC, offering all the benefits without any of the drawbacks. Because dye detection occurs quickly—within 15 minutes of peripheral venous infusion—and lasts for several hours—IOC-ICG is a good choice for laparoscopic cholecystectomies. The method makes it easier to divide Calot's triangle and permits real-time bile duct exploration. As of right now, no one has reported experiencing BDI during IOC-ICG (9).
A novel technique, intraoperative fluorescent cholangiogram by using indocyanine green (ICG), has been proposed as a replacement for intraoperative visualization of the extrahepatic biliary system. This new technique has proven feasible, faster and safer than conventional X-ray cholangiography (3).
For this reason, fluorescence cholangiography functions similarly to traditional X-ray cholangiography. Nonetheless, it has more benefits, including the ability to clarify the dissection in real time, the avoidance of radiation, and the fact that the surgeon can complete the procedure alone without the assistance of other personnel. While specialized equipment is needed, it can be readily included into a conventional laparoscopy tower (9).
Crucially, it has not been demonstrated that fluorescence cholangiography with ICG is an accurate method of identifying common bile duct stones. Consequently, in situations when common bile duct obstruction is a concern, it shouldn't take the place of traditional intraoperative cholangiography (10).
In this prospective study, we report the use of intravenous indocyanine green (ICG) injection prior to laparoscopic cholecystectomy to demarcate extra hepatic biliary anatomy. We compare the results with traditional visual inspection prior to dissection around Calot's triangle and with conventional intraoperative cholangiography (IOC) following dissection around Calot's triangle in the same patient.
Quaresima et al. (2) believe that intravenous administration of ICG is the optimal approach because the Veress technique may cause ICG to disseminate throughout the abdominal cavity, which could impact the results linked to biliary tree imaging. Furthermore, ICG may not enter the common bile duct when a gallstone impacts at the infundibulum, which affects the visibility of the gallstone.
There were fifty patients in our study. Eighty percent of the participants were female, with a mean age of 40.5 ± 11.5 and a mean BMI of 31.92 ± 3.94. Preoperative laboratory tests were performed normally. Every participant's liver and kidneys were operating normally. Nobody experienced obstructive jaundice or an acute bout.
The conventional IOC took between 15 and 45 minutes, while the overall operating time varied from 45 to 180 minutes. This indicates that the conventional IOC caused a delay in the overall operating time.
Our results are in agreement with Quaresima et al. (2), who reported that conventional IOC delayed the total operative time 10 to 40 minutes.
Before dissecting the area around Calot's triangle, we discovered that ICG dye was successful in identifying the cystic duct (CD) in 60% of cases, the cystic-hepatic junction (CHJ) in 52% of cases, the common bile duct (CBD) in 76% of cases, the common hepatic duct (CHD) in 54% of cases, and the analyzability rate (i.e., the synchronous clarifying of the cystic duct, cystic-hepatic junction, and common bile duct) in 52% of cases. By conventional visual inspection, however, the cystic duct (CD) was visualized in 18% of cases, the cystic-hepatic junction (CHJ) in 16% of cases, the common bile duct (CHD) in 14% of cases, and the analyzability rate in 16% of cases, which is statistically significant.
The findings of this study are consistent with those of Prevot et al. (9), who found that by conventional visual inspection, CD was visualized in 26% of cases, CHJ in 26% of cases, CBD in 48% of cases, CHD in 9% of cases, and analyzability rate in 26% of cases. In contrast, CD was delineated in 61% of cases, CHJ in 48% of cases, CBD in 74% of cases, CHD in 13% of cases, and analyzability rate in 48% of cases.
Furthermore, our findings concur with those of Quaresima et al. (2), who indicated that CD, CHJ, CHD, and CBD were statistically different when viewed by ICG as opposed to eye inspection.
During laparoscopic cholecystectomy, conventional IOC can be utilized to detect anatomic differences linked to a risk of biliary injury, diagnose BDI quickly, and ensure that no undetected CBD stones are present. Furthermore, regular conventional IOC has been shown by Flum et al. (11) to be facilitate preventing BDI (12).
It's unknown if traditional IOC is beneficial in preventing BDI. Additionally, it was noted that the operating surgeon did not identify 45 to 50% of BDIs that were apparent on an intraoperative cholangiogram as such (13).
Only 28% of all BDIs could be diagnosed with regular use of conventional IOC, according to Nuzzo et al. (14,15).
In our study, all 50 patients had IOC using ICG; however, only 41 patients received conventional IOC due to procedural failure for a variety of reasons, including stone impaction in the CD, extremely thin CD that needed to be cannulated, or sessile gall bladders with either no CD or a very short CD.
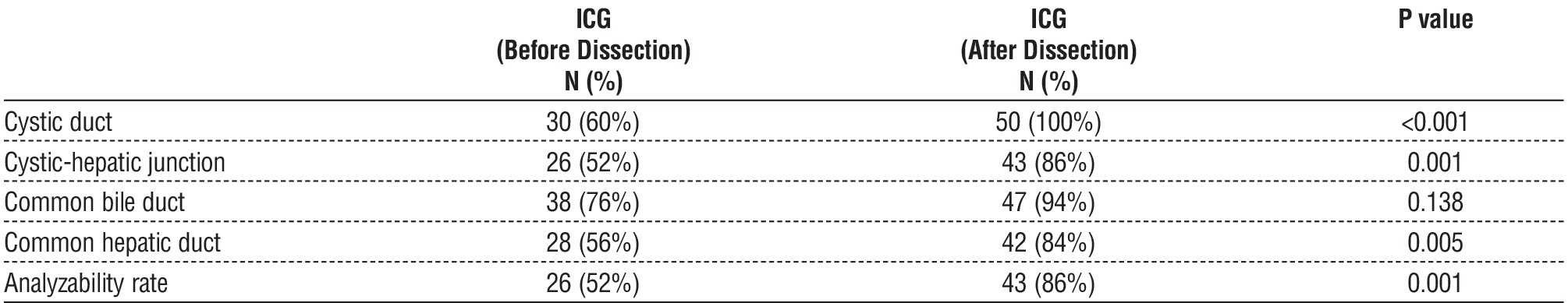
Following dissection around Calot's triangle, all CD cases (100%) and all CHJ cases (86%), CBD cases (94%), CHD cases (84%), and analyzability rate cases (86%), were delineated by ICG following dissection, whereas all structures were delineated by appropriate IOC in 82% of instances.
We found no statistically significant difference between traditional IOC following dissection and intraoperative cholangiography employing ICG in our investigation.
These findings concur with those of Lehrskov et al. (3), who found no discernible difference in the extrahepatic biliary architecture visualization between the fluorescence ICG and X-ray cholangiography groups.
These findings also support a research by Dip et al. (16), which found no statistically significant difference in the identification of bile structures between IOC employing X-ray cholangiography and ICG.
Prevot et al. (9) found that the mean analyzability rate was 74% for IOC utilizing ICG after dissection and 70% for traditional IOC (p=0.03), which is in contrast to our data.
In our investigation, CD increased from 60% to 100%, CHJ from 52% to 86%, and CHD from 56% to 84% utilizing ICG before and after dissection of Calot's triangle visualization. These increases were statistically significant. There was no statistically significant increase in CBD visualization from 76% to 94%.
Iatrogenic biliary injury is the nightmare sequalae during cholecystectomy. Iatrogenic biliary injury incidence is between 0.08 to 0.3% (17,18). That low rate has to be unconsidered in contrast to increased incidence of cholecystectomies (19,20).
Our findings concur with those of Quaresima et al. (2), who found that CD visualization was not statistically significant, but that CHJ, CHD, and CBD visualization using ICG before and after Calot's triangle dissection was.
CONCLUSION
Our results suggest that IOC using ICG is a feasible, safe, fast and more effective technique than conventional IOC for biliary tree visualization before and after dissection of Calot’s triangle and may constitute a powerful diagnostic test for delineation of extrahepatic bile ducts in laparoscopic cholecystectomy. One important consideration is that fluorescent ICG cholangiography has not been shown to effectively in identifying stones of common bile duct. Therefore, it should not replace conventional X-ray cholangiography in cases with concern for common bile duct obstruction. Lastly, in the presence of inflammatory changes, the identification of the biliary tract by means of ICG alone might be limited. In that case, a combined ICG for identification of the cystic duct followed by conventional IOC for verification of cystic duct and CBD junction should be considered.
Acknowledgements
The authors thank the faculty of medicine, Cairo University and Theodor Bilharz Research Institute for providing facilities and software support for this research. Mahmoud Ahmed Nouh is grateful for the infinite and selfless support from Ayman Salah Helmy, Mohamed Abbas, Maged Nasr, M. Emad Esmat, Sameh Adel Aziz and Hesham A. Elmeligy.
Conflict of interest: None.
Funding: None.
REFERENCES
1. Kaneko J, Ishizawa T, Masuda K, Kawaguchi Y, Aoki T, Sakamoto Y, et al. Indocyanine green reinjection technique for use in fluorescent angiography concomitant with cholangiography during laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2012; 22(4):341-4.
2. Quaresima S, Balla A, Palmieri L, Seitaj A, Fingerhut A, Ursi P, et al. Routine near infra-red indocyanine green fluorescent cholangiography versus intraoperative cholangiography during laparoscopic cholecystectomy: a case-matched comparison. Surg Endosc. 2020;34(5):1959-1967.
3. Lehrskov LL, Westen M, Larsen SS, Jensen AB, Kristensen BB, Bisgaard T. Fluorescence or X-ray cholangiography in elective laparoscopic cholecystectomy: a randomized clinical trial. Br J Surg. 2020;107(6):655-661.
4. Liu YY, Liao CH, Diana M, Wang SY, Kong SH, Yeh CN, et al. Near-infrared cholecystocholangiography with direct intragallbladder indocyanine green injection: preliminary clinical results. Surg Endosc. 2018;32(3):1506-1514.
5. Ambe PC, Plambeck J, Fernandez-Jesberg V, Zarras K. The role of indocyanine green fluoroscopy for intraoperative bile duct visualization during laparoscopic cholecystectomy: an observational cohort study in 70 patients. Patient Saf Surg. 2019;13:2.
6. Van Den Bos J, Schols RM, Luyer MD, van Dam RM, Vahrmeijer AL, Meijerink WJ, et al. Near-infrared fluorescence cholangiography assisted laparoscopic cholecystectomy versus conventional laparoscopic cholecystectomy (FALCON trial): study protocol for a multicentre randomized controlled trial. BMJ Open. 2016;6(8): e011668.
7. Boni L, David G, Mangano A, Dionigi G, Rausei S, Spampatti S, et al. Clinical applications of indocyanine green (ICG) enhanced fluorescence in laparoscopic surgery. Surg Endosc. 2015;29(7): 2046-55.
8. Koong JK, Ng GH, Ramayah K, Koh PS, Yoong BK. Early identification of the critical view of safety in laparoscopic cholecystectomy using indocyanine green fluorescence cholangiography: A randomized controlled study. Asian J Surg. 2021;44(3):537-543.
9. Prevot F, Rebibo L, Cosse C, Browet F, Sabbagh C, Regimbeau JM. Effectiveness of intraoperative cholangiography using indocyanine green (versus contrast fluid) for the correct assessment of extra-hepatic bile ducts during day-case laparoscopic cholecystectomy. J Gastrointest Surg. 2014;18(8):1462-8.
10. Vlek SL, Van Dam DA, Rubinstein SM, de Lange-de Klerk ES, Schoonmade LJ, Tuynman JB, et al. Biliary tract visualization using near-infrared imaging with indocyanine green during laparoscopic cholecystectomy: results of a systematic review. Surg Endosc. 2017; 31(7):2731-2742.
11. Flum DR, Dellinger EP, Cheadle A, Chan L, Koepsell T. Intraoperative cholangiography and risk of common bile duct injury during cholecystectomy. JAMA. 2003;289(13):1639-44.
12. Michael Brunt L, Deziel DJ, Telem DA, Strasberg SM, Aggarwal R, Asbun H, et al. Safe cholecystectomy multi-society practice guideline and state-of-the-art consensus conference on prevention of bile duct injury during cholecystectomy. Surg Endosc. 2020;34(7):2827-2855.
13. Christou N, Roux-David A, Naumann DN, Bouvier S, Rivaille T, Derbal S, et al. Bile duct injury during cholecystectomy: necessity to learn how to do and interpret intraoperative cholangiography. Front Med (Lausanne). 2021;8:637987.
14. Nuzzo G, Giuliante F, Giovannini I, Ardito F, D’Acapito F, Vellone M, et al. Bile duct injury during laparoscopic cholecystectomy: results of an Italian national survey on 56,591 cholecystectomies. Arch Surg. 2005;140(10):986-92.
15. Van Manen L, Handgraaf HJ, Diana M, Dijkstra J, Ishizawa T, Vahrmeijer AL, et al. A practical guide for the use of indocyanine green and methylene blue in fluorescence-guided abdominal surgery. J Surg Oncol. 2018;118(2):283-300.
16. Dip F, Roy M, Menzo EL, Simpfendorfer C, Szomstein S, Rosenthal RJ. Routine use of fluorescent incisionless cholangiography as a new imaging modality during laparoscopic cholecystectomy. Surg Endosc. 2015;29(6):1621-6.
17. Halbert C, Pagkratis S, Yang J, Meng Z, Altieri MS, Parikh P, et al. Beyond the learning curve: incidence of bile duct injuries following laparoscopic cholecystectomy normalize to open in the modern era. Surg Endosc. 2016;30(6):2239-43.
18. Rystedt J, Lindell G, Montgomery A. Bile duct injuries associated with 55,134 cholecystectomies: treatment and outcome from a national perspective. World J Surg. 2016;40(1):73-80.
19. Bektas H, Schrem H, Winny M, Klempnauer J. Surgical treatment and outcome of iatrogenic bile duct lesions after cholecystectomy and the impact of different clinical classification systems. Br J Surg. 2007;94(9):1119-27.
20. Ausania F, Holmes LR, Ausania F, Iype S, Ricci P, White SA. Intraoperative cholangiography in the laparoscopic cholecystectomy era: Why are we still debating? Surg Endosc. 2012;26:1193-200.
Full Text Sources:
Abstract:
Views: 3636

For Authors
Journal Subscriptions

Jun 2025
Supplements
Instructions for authors
Online submission
Contact
e-ISSN: 2601 - 1700 (online)
ISSN-L: 2559 - 723X
Journal Abbreviation: Surg. Gastroenterol. Oncol.
Surgery, Gastroenterology and Oncology (SGO) is indexed in:
- SCOPUS
- EBSCO
- DOI/Crossref
- Google Scholar
- SCImago
- Harvard Library
- Open Academic Journals Index (OAJI)
Surgery, Gastroenterology and Oncology (SGO) is an open-access, peer-reviewed online journal published by Celsius Publishing House. The journal allows readers to read, download, copy, distribute, print, search, or link to the full text of its articles.
Time to first editorial decision: 25 days
Rejection rate: 61%
CiteScore: 0.2

Meetings and Courses in 2025
Meetings and Courses in 2024
Meetings and Courses in 2023
Meetings and Courses in 2022
Meetings and Courses in 2021
Meetings and Courses in 2020
Meetings and Courses in 2019
Verona expert meeting 2019

Surgery, Gastroenterology and Oncology applies the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits readers to copy and redistribute the material in any medium or format, remix, adapt, build upon the published works non-commercially, and license the derivative works on different terms, provided the original material is properly cited and the use is non-commercial. Please see: https://creativecommons.org/licenses/by-nc/4.0/
Publisher’s Note:
The opinions, statements, and data contained in article are solely those of the authors and not of Surgery, Gastroenterology and Oncology journal or the editors. Publisher and the editors disclaim responsibility for any damage resulting from any ideas, instructions, methods, or products referred to in the content.
 IASGO Society News
IASGO Society News