Surgery, Gastroenterology and Oncology
|
|
Abstract
Introduction: Pancreatic neuroendocrine tumors (PNETs) are uncommon, representing fewer than 5% of all pancreatic malignancies. They are a diverse group of tumors with different biological behaviors, clinical features, and prognostic outcomes. Reported 5-year survival varies widely, from 25% up to 100% in some series. Aim: Evaluate recurrence
patterns, risk factors, and their influence on disease free survival (DFS) and overall survival (OS).
Methods: Retrospective review of 87 patients undergoing curative surgical resection for PNETs in a tertiary referral center (2008–2022).
Results: The cohort included 47,1% males and 52,9% females, with mean age of 55,68 years (13-77 years). Recurrence occurred in 16 patients (18.4%), with a median time to relapse of 20.5 months (range 3–107) during a median follow-up of 57 months (range 7–175). The liver was the most frequent site of recurrence, and chemotherapy was the most common treatment. Median OS was 75 months. Survival among patients with recurrence (96.6%) was not significantly different from those without recurrence (100%), with only three deaths reported post-recurrence. Significant predictors of relapse in univariate analyses were tumor size, stage, Ki-67 index, necrosis, perineural growth, venous involvement, and lymphatic invasion.
Conclusions: Several pathological features, including tumor size, staging parameters,
proliferative index, and patterns of invasion, are strongly associated with recurrence after surgical removal of PNETs. Nevertheless, OS in patients experiencing recurrence remains comparable to those without relapse.
Introduction
PNETs are rare, accounting for less than 5% of pancreatic tumors (1–3). Although infrequent, their incidence has risen in recent decades (4–6). These tumors vary considerably in terms of biological activity, clinical presentation, and long-term outcome (7,8).
They are categorized as functioning or non-functioning depending on hormone secretion. Most cases (50–90%) are non-functioning (7). Functioning tumors, such as insulinomas or gastrinomas, cause specific clinical syndromes and tend to be detected earlier. Non-functioning lesions often present late with vague symptoms such as pain, weight loss, or jaundice (5). With the growing use of advanced imaging, incidental diagnoses are increasingly common (5,6).
Despite progress in systemic therapy, surgery remains the only curative option (9). Still, recurrence after resection may reach up to 35% and has a negative effect on survival and quality of life (10-13). Five-year survival has been reported to range broadly between 25% and 100% (7). This study aims to determine recurrence frequency, patterns, and prognostic predictors in surgically treated PNETs, as well as their impact on survival.
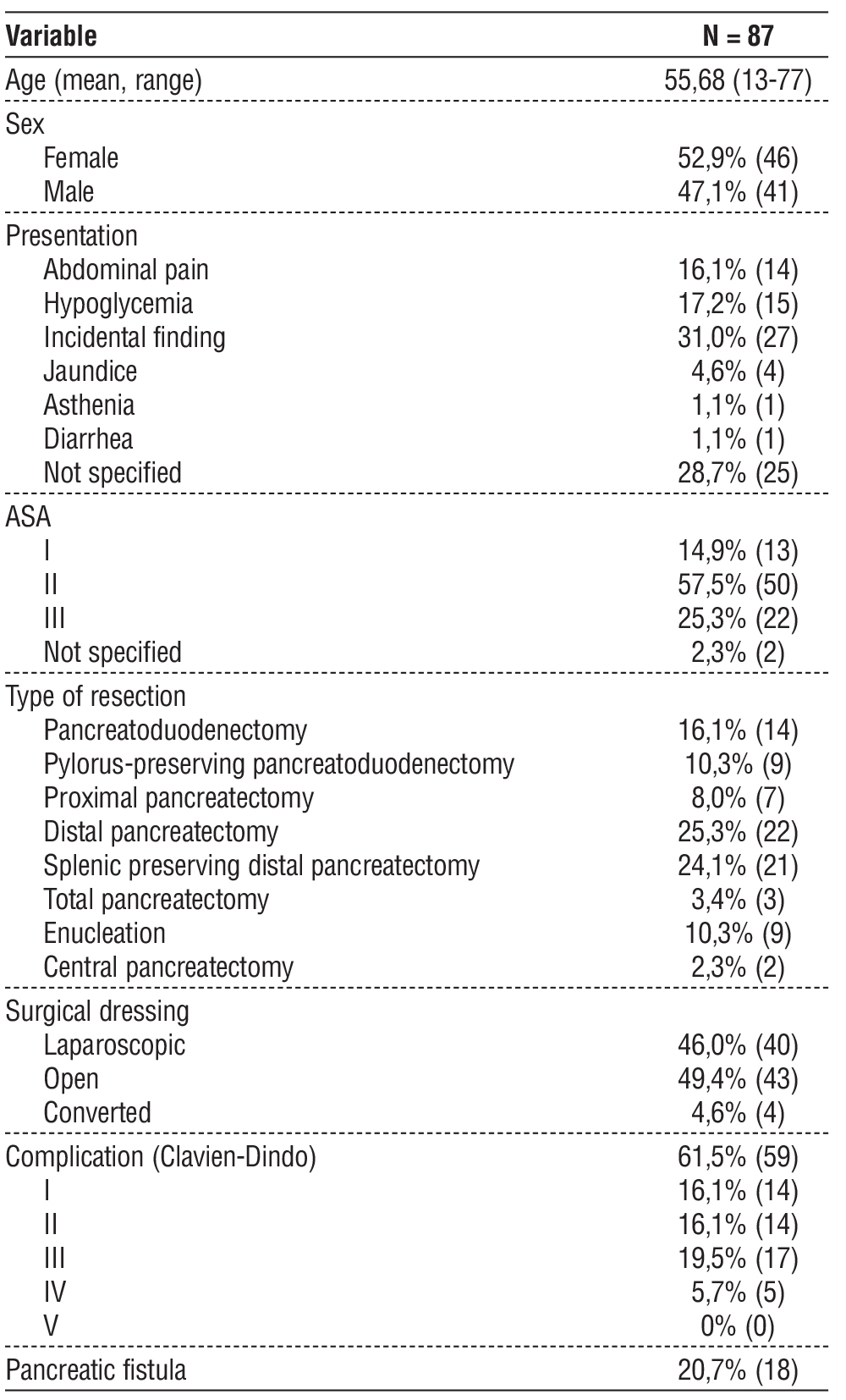
Table 1 - Patients clinical data
METHODS
The study was performed at the Department of General Surgery, in a tertiary hospital center. In this study, we retrospectively examined our database of patients with PNETs who underwent surgical resection between 2008 and Dec. 2022 (n= 87).
Exclusion Criteria
Patients with incomplete clinical/follow-up data were excluded. A detailed retrospective review of clinicopathologic data on patients with primary PNETs was carried out, based on electronic patient records, specialty consultation files, tumor registry and pathology archives. All available tumor slides were reviewed and subtyped by a pathologist. Clinicopathologic data, tumor recurrence and patient survival were analysed.
Our follow-up protocol was every 6 months for the first 3 years and annually thereafter and consisted in physical examination, imaging and chromogranin A testing.
Recurrence was considered when a lesion in the surgical bed, nodal or at distant was visible on imaging.
Statistical Analysis
Data was analysed by using SPSS (version 30.0). Descriptive statistics were generated for all measures. Chi-Square Test was used to compare categorical variables and Wilcoxon Rank Sum Test was used for continuous variables.
Univariate and multivariate analysis were performed with Cox proportional hazard models, and survival analyses were performed using Kaplan-Meier Curves with Log Rank test for significance.
Differences were considered to be statistically significant at p < 0.05.
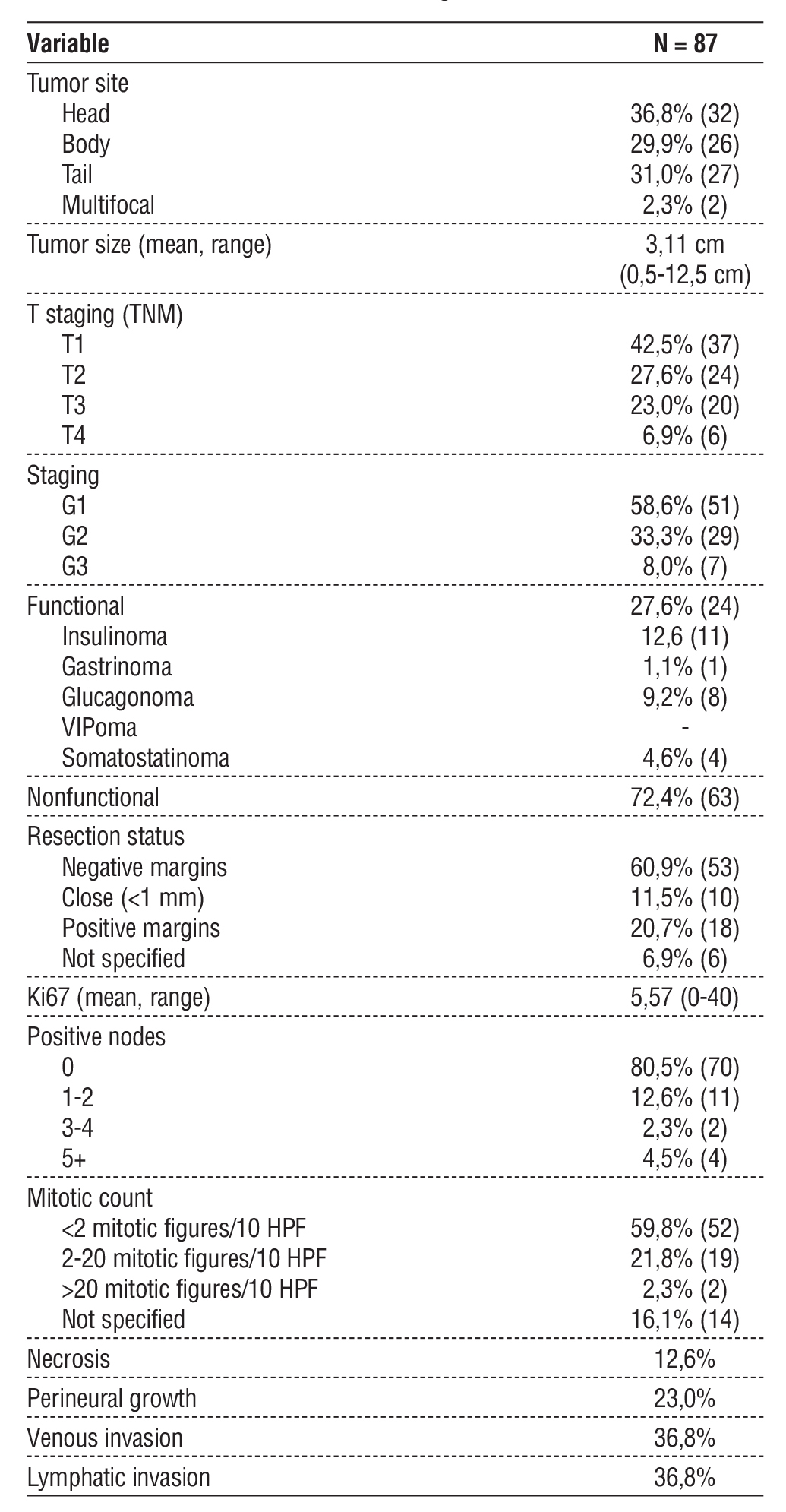
Table 2 - Pathologic data
RESULTS
Eighty-seven patients with PNETs underwent surgical resection. The detailed patient characteristics are listed in table 1. 47,1% (n=41) were male and 52,9% (n=46) female, with mean age of 55,68 years (13-77 years). In the majority of the cases, it was an incidental finding. 25,3% (n=22) were submitted to distal pancreatectomy and 24,1% (n=21) to splenic preserving distal pancreatectomy, those were the most common procedures. 16,1% (n=14) had pancreatoduodenec-tomy, 14,9% (n=13) enucleation, 13,8% (n=12) pylorus-preserving pancreatoduodenectomy, and a minority of cases had central pancreatectomy (2,3%) or required a total pancreatectomy (3,4%).
61,5% (n=59) had complications, but mostly Clavien-Dindo I-III. 20,7% (n=18) had pancreatic fistula.
Table 2 present the pathologic results. 72,4% (n=63) of the tumors were nonfunctional. The most common functional tumor type was insulinoma (n=11). In terms of tumor site, 36,8% (n=32) were located in the head, 31% (n=27) in the tail and 29,9% (n=26) in the body. Only 2 cases were multifocal.
The mean tumor size was 3,11 cm (range 0,5-12,5 cm). 42,5% (n=37) were at stage T1, 27,6% (n=24) at stage T2, 23,0% (n=20) at stage T3 and the minority at stage T4 (6,9%). 58,6% (n=51) were classified as G1, 33,3% (n=29) were G2 and 8,0% (n=7) G3.
The majority 60,9% (n=53) had R0 resection, but 20,7% (n=18) had positive margins.
The Ki-67 mean was 5,57 (range 0-40).
Table 3 specify the follow-up, recurrence rate, site and time to recurrence, DFS and OS. Table 4 analyse the clinicopathologic characteristic of the patients that presented recurrence, specifying the site and time to recurrence, the treatment and OS of these patients.
Table 3 – Follow-up. recurrence and overall survival
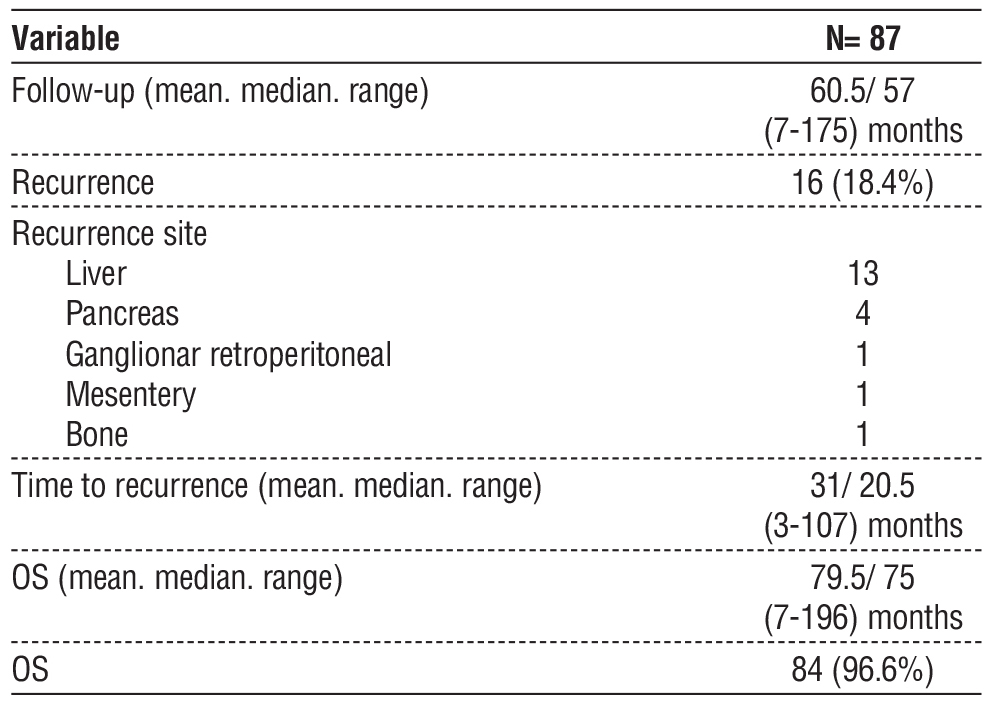
There were 16 (18,4%) recurrences, with median time to recurrence of 20,5 months (3-107 months), and a median follow-up of 57 months (range 7-175 months). The most common site of recurrence was the liver. The most common treatment of recurrences was chemotherapy.
Median DFS was 62 months (3-196 months) and median OS was 75 months (7-196 months). OS for those with and without recurrence was 96,6% and 100%, with only 3 deaths after recurrence (table 4). This was not considered a significantly different OS for those with or without recurrence (fig. 1). These patients were at stage T3-4 at the time of the diagnosis, all had R0 resection and presented recurrence to the liver in 7-11 months after surgery with an OS between 12 and 27 months.
Table 4 – Clinicopathologic analysis of the patients with recurrence
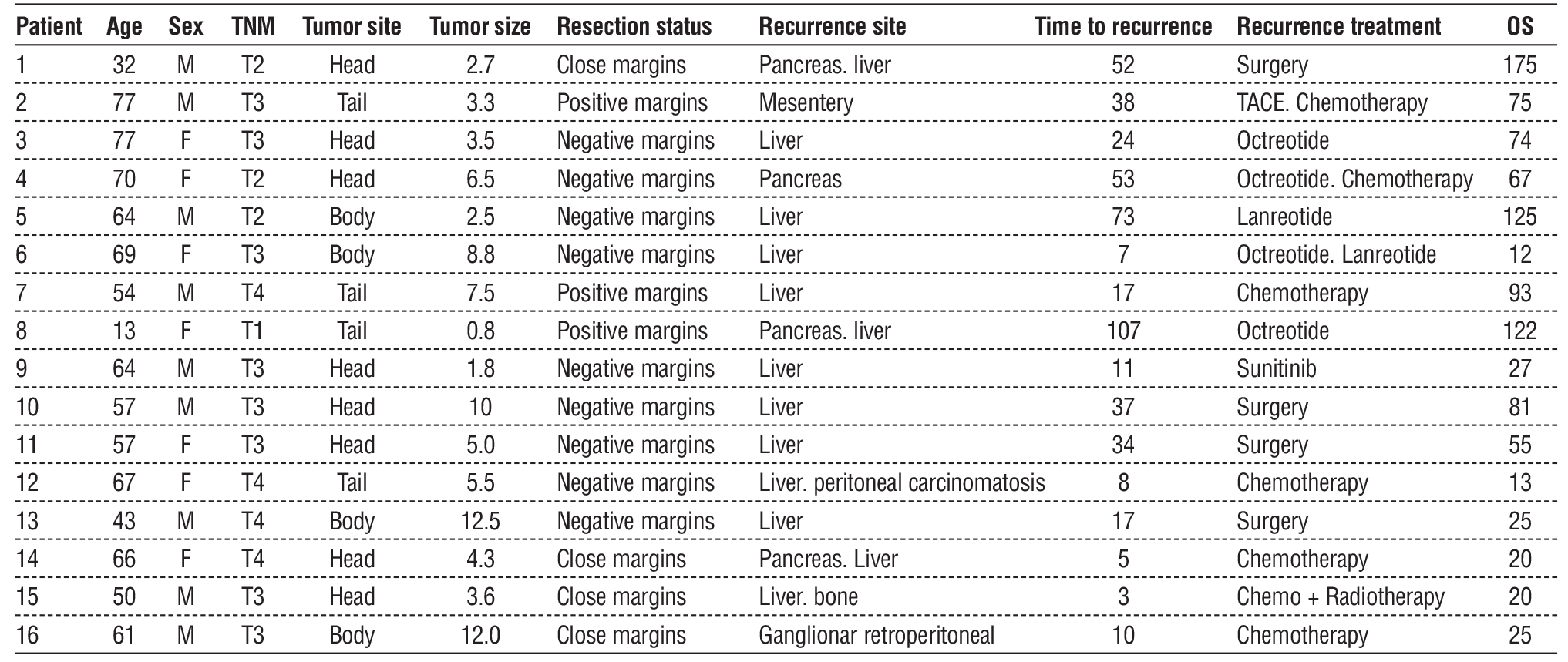
Figure 1 – Overall survival stratified by disease recurrence
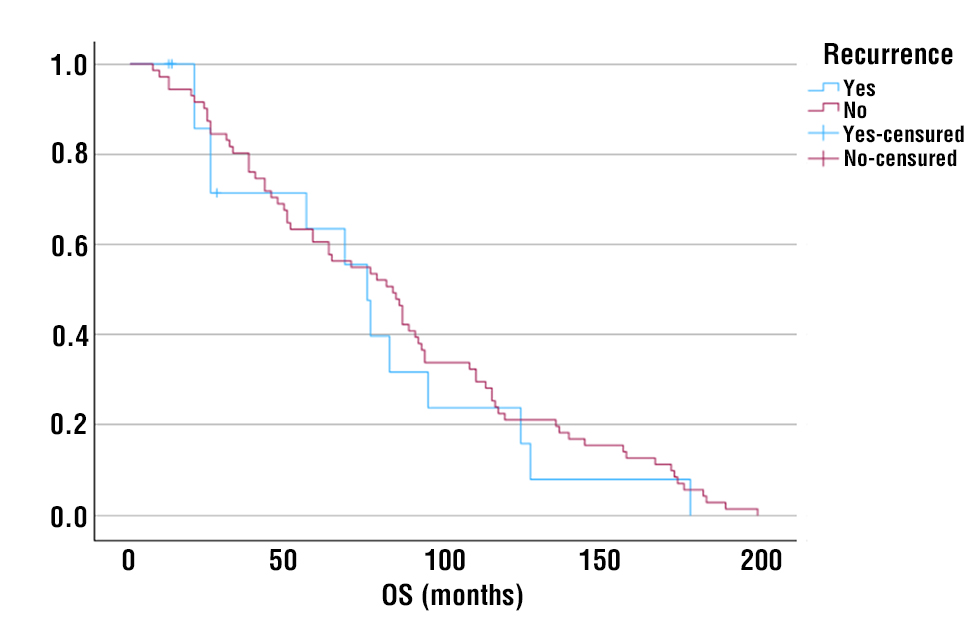
Table 5 describes the univariate and multivariate analysis results highlining the variables associated with a higher risk of recurrence.
Table 5 – Univariate and multivariate analysis of predictors of recurrence
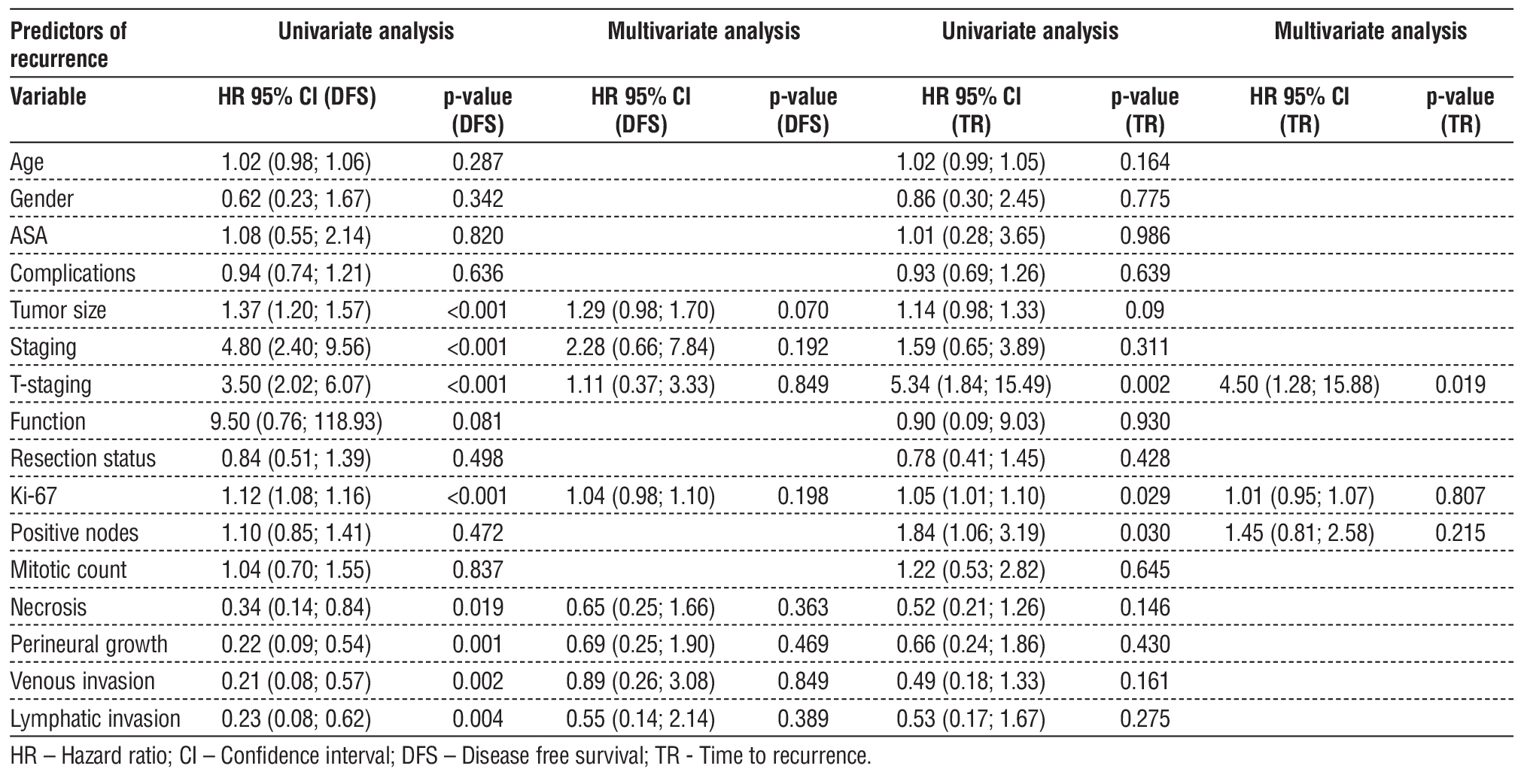
On univariate analysis, variables adversely impacting DFS were tumor size, staging, T-staging, Ki-67, necrosis, perineural growth, venous invasion, and lymphatic invasion, considered statistically significant predictors of recurrence. Figures 2-9 respectively show DFS stratified by tumor size, staging, T-staging, Ki-67, necrosis, perineural growth, venous invasion and lymphatic invasion, graphically highlighting the effect of these variables in DFS.
Concerning the time to recurrence, on univariate analysis, T-staging, Ki-67 and positive nodes were the variables considered statistically significant.
On multivariate analysis, in relation to DFS no variables were identified as statistically significant, but in relation to the time to recurrence, T-staging was considered a statistically significant predictor of an early recurrence.
Discussion
Recent years have shown a growing number of incidental PNET diagnoses (4). In our series, 31% were found unintentionally. Data suggest that tumors <2 cm have increased in incidence by more than sevenfold over two decades (4).
Surgical resection is standard for localized disease, though pancreatic surgery carries notable morbidity and non-trivial mortality (7). In our study, complications occurred in 61.5%, mostly Clavien-Dindo I–III, with 20.7% developing pancreatic fistulas.
Management of small, non-functioning PNETs remains controversial. European Neuroendocrine Tumor Society (ENETS) and North American Neuroendocrine Tumor Society (NANETS) recommend surveillance for tumors <2 cm, whereas National Comprehensive Cancer Network (NCCN) guidelines suggest observation mainly for low-grade lesions <1 cm (7). The optimal threshold distinguishing indolent from aggressive tumors may lie between 1.5–2 cm (15). Since PNETs are rare, their natural history remains insufficiently understood, complicating predictions of malignant potential (14).
Although some series report recurrence in <10% of resected tumors <2 cm (7), others describe recurrence rates up to 35% overall (10–13). Our cohort showed an 18.4% recurrence rate. While generally considered indolent, PNETs are prone to relapse, which significantly influences prognosis. Understanding timing, distribution, and predictors of recurrence is essential for tailoring follow-up and guiding adjuvant treatment strategies (7,8,14).
Risk factors for aggressive disease include larger tumor size, vascular and lymphatic invasion, nodal or distant metastases at presentation, and higher grade (1,2,13,16–18). Patients with nodal involvement show reduced 5-year disease-free survival compared to node-negative patients (1,2,4,16,18,19). Other prognostic determinants include WHO grade, Ki-67 index, mitotic rate, degree of differentiation, and functionality (7,16).
Predictors consistently reported in literature are size, lymph node metastases (×5 increased recurrence risk), Ki-67 index, vascular/perineural invasion, necrosis, grade G3 tumors, and positive surgical margins (13-22). Surveillance strategies should therefore be tailored to high-risk individuals, as recurrence may appear late (14).
In our study, variables with significant adverse impact on DFS were tumor size, staging, T-staging, Ki-67, necrosis, perineural growth, venous invasion, and lymphatic invasion. On multivariate analysis, T-staging was also considered a statistically significant predictor of an early recurrence.
The most common site of relapse is the liver, accounting for over half of cases, followed by pancreatic remnant and lymph nodes (2,4,8,16). In our series, liver recurrence predominated, with chemotherapy being the most frequent salvage treatment.
Median OS was 75 months. Survival rates were high in both groups - 96.6% in patients with recurrence and 100% in those without, with only three deaths occurring after relapse.
Conclusion
PNET recurrence after curative resection is relatively common, predominantly affecting the liver. Identification of risk factors - such as tumor size, pathological staging, proliferation index, and invasive features - is critical to stratify patients for intensive monitoring or adjuvant treatment. Despite recurrence, OS outcomes remain favorable. Given the rarity and heterogeneity of PNETs, additional multicenter studies are required to refine prognostic markers and optimize follow-up strategies.
Author’s Contributions
Conceptualization: EC, RRD. Data curation: EC, RRD, SR, MA, JL. Methodology: EC, RRD, MA. Visualization: EC, RRD, MA. Writing - original draft: EC, RRD. Writing - review & editing: SR, MA, JL, HC, LG, SC.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
References
1. Zaidi MY, Lopez-Aguiar AG, Switchenko JM, Lipscomb J, Andreasi V, Partelli S et al. A Novel Validated Recurrence Risk Score to Guide a Pragmatic Surveillance Strategy After Resection of Pancreatic Neuroendocrine Tumors: An International Study of 1006 Patients. Ann Surg. 2019;270(3):422-433.
2. Chouliaras K, Newman NA, Shukla M, Swett KR, Levine EA, Sham J, et al. Analysis of recurrence after the resection of pancreatic neuroendocrine tumors. J Surg Oncol. 2018;118(3):416-421.
3. Amin S, Kim MK. Islet cell tumors of the pancreas. Gastroenterol ClinNorth Am. 2016;45:83-100.
4. Kuo EJ, Salem RR. Population-level analysis of pancreatic neuro-endocrine tumors 2 cm or less in size. Ann Surg Oncol. 2013;(20): 2815-2821.
5. Xu X, Honda K, Miura N, Hori S, Le Blanc S, Bergmann F et al. Actinin-4 splice variant - a complementary diagnostic and prognostic marker of pancreatic neuroendocrine neoplasms. J Cancer. 2020;11(8):2318-2328.
6. Li WX, Miao F, Xu XQ, Zhang J, Wu ZY, Chen KM, et al. Pancreatic Neuroendocrine Neoplasms: CT Spectral Imaging in Grading. Acad Radiol. 2021;28(2):208-216.
7. Assi HA, Mukherjee S, Kunz PL, Machiorlatti M, Vesely S, Pareek V, et al. Surgery Versus Surveillance for Well-Differentiated, Non-functional Pancreatic Neuroendocrine Tumors: An 11-Year Analysis of the National Cancer Database. Oncologist. 2020; 25(2):e276-e283.
8. Dong DH, Zhang XF, Lopez-Aguiar AG, Poultsides G, Makris E, Rocha F, et al. Resection of pancreatic neuroendocrine tumors: defining patterns and time course of recurrence. HPB (Oxford). 2020;22(2):215-223.
9. Bidani K, Marinovic AG, Moond V, Harne P, Broder A, Thosani N. Treatment of Pancreatic Neuroendocrine Tumors: Beyond Traditional Surgery and Targeted Therapy. J Clin Med. 2025;14(10):3389.
10. Fischer L, Bergmann F, Schimmack S, Hinz U, Priess S, Muller-Stich BP et al. Outcome of surgery for pancreatic neuroendocrine neoplasms. Br J Surg. 2014;101(11):1405-12.
11. Wong J, Fulp WJ, Strosberg JR, Kvols LK, Centeno BA, Hodul PJ. Predictors of lymph node metastases and impact on survival in resected pancreatic neuroendocrine tumors: a single-center experience. Am J Surg. 2014;208(5):775-780.
12. Wei IH, Harmon CM, Arcerito M, Cheng DF, Minter RM, Simeone DM. Tumor-associated macrophages are a useful biomarker to predict recurrence after surgical resection of nonfunctional pancreatic neuroendocrine tumors. Ann Surg. 2014;260(6):1088-94.
13. Hamilton NA, Liu TC, Cavatiao A, Mawad K, Chen L, Strasberg SS et al. Ki-67 predicts disease recurrence and poor prognosis in pancreatic neuroendocrine neoplasms. Surgery. 2012;152(1):107-13.
14. Kim H, Song KB, Hwang DW, Lee JH, Alshammary S, Kim SC. Time-trend and recurrence analysis of pancreatic neuroendocrine tumors. Endocr Connect. 2019;8(7):1052-1060.
15. Regenet N, Carrere N, Boulanger G, de Calan L, Humeau M, Arnault V et al. Is the 2-cm size cutoff relevant for small nonfunctioning pancreatic neuroendocrine tumors: A French multicenter study. Surgery. 2016;159(3):901-7.
16. Pulvirenti A, Pea A, Chang DK, Jamieson NB. Clinical and Molecular Risk Factors for Recurrence Following Radical Surgery of Well-Differentiated Pancreatic Neuroendocrine Tumors. Front Med (Lausanne). 2020;7:385.
17. Mukkala AN, Ray S, Bevacqua D, McGilvray I, Sapisochin G, Moulton CA et al. Disease-free survival after pancreatectomy for pancreatic neuroendocrine tumors: A 17-year single-center experience of 223 patients. J Gastrointest Surg. 2024;28(9):1485-1492.
18. Zhang Z, Liu M, Ji S, Luo G, Xu W, Liu W et al. Prognostic Value and Clinical Predictors of Lymph Node Metastases in Pancreatic Neuroendocrine Tumors. Pancreas. 2020;49(3):381-386.
19. Boninsegna L, Panzuto F, Partelli S, Capelli P, Delle Fave G, Bettini R et al. Malignant pancreatic neuroendocrine tumour: lymph node ratio and Ki67 are predictors of recurrence after curative resections. Eur J Cancer. 2012;48(11):1608-15.
20. Casadei R, Ricci C, Pezzilli R, Campana D, Tomassetti P, Calculli L et al. Are there prognostic factors related to recurrence in pancreatic endocrine tumors? Pancreatology. 2010;10(1):33-8.
21. Genç CG, Jilesen AP, Partelli S, Falconi M, Muffatti F, van Kemenade FJ, et al. A New Scoring System to Predict Recurrent Disease in Grade 1 and 2 Nonfunctional Pancreatic Neuroendocrine Tumors. Ann Surg. 2018;267(6):1148-1154.
22. Nanno Y, Toyama H, Otani K, Asari S, Goto T, Terai S, et al. Microscopic venous invasion in patients with pancreatic neuro-endocrine tumor as a potential predictor of postoperative recurrence. Pancreatology. 2016;16(5):882-7.
23. Landoni L, Marchegiani G, Pollini T, Cingarlini S, D'Onofrio M, Capelli P et al. The evolution of surgical strategies for pancreatic neuro-endocrine tumors (pan-nens): time-trend and outcome analysis from 587 consecutive resections at a high-volume institution. Ann Surg. 2019;269(4):725-732.
24. Xu Han M, Xuefeng DJ. Clinicopathological characteristics and prognosis related factors of resectable pnets. Pancreas. 2014; 43(4):526-31.
25. Sun HT, Zhang SL, Liu K, Zhou JJ, Wang XX, Shen TT, et al. MRI-based nomogram estimates the risk of recurrence of primary nonmetastatic pancreatic neuroendocrine tumors after curative resection. J Magn Reson Imaging. 2019;50(2):397-409.
26. Partelli S, Bartsch DK, Capdevila J, Chen J, Knigge U, Niederle B, et al. ENETS Consensus Guidelines for Standard of Care in Neuroendocrine Tumours: Surgery for Small Intestinal and Pancreatic Neuroendocrine Tumours. Neuroendocrinology. 2017; 105(3):255-265.
Full Text Sources:
Abstract:
Views: 19

For Authors
Journal Subscriptions

Sept 2025
Supplements
Instructions for authors
Online submission
Contact
e-ISSN: 2601 - 1700 (online)
ISSN-L: 2559 - 723X
Journal Abbreviation: Surg. Gastroenterol. Oncol.
Surgery, Gastroenterology and Oncology (SGO) is indexed in:
- SCOPUS
- EBSCO
- DOI/Crossref
- Google Scholar
- SCImago
- Harvard Library
- Open Academic Journals Index (OAJI)
Surgery, Gastroenterology and Oncology (SGO) is an open-access, peer-reviewed online journal published by Celsius Publishing House. The journal allows readers to read, download, copy, distribute, print, search, or link to the full text of its articles.
Time to first editorial decision: 25 days
Rejection rate: 61%
CiteScore: 0.2

Meetings and Courses in 2025
Meetings and Courses in 2024
Meetings and Courses in 2023
Meetings and Courses in 2022
Meetings and Courses in 2021
Meetings and Courses in 2020
Meetings and Courses in 2019
Verona expert meeting 2019

Surgery, Gastroenterology and Oncology applies the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits readers to copy and redistribute the material in any medium or format, remix, adapt, build upon the published works non-commercially, and license the derivative works on different terms, provided the original material is properly cited and the use is non-commercial. Please see: https://creativecommons.org/licenses/by-nc/4.0/
Publisher’s Note:
The opinions, statements, and data contained in article are solely those of the authors and not of Surgery, Gastroenterology and Oncology journal or the editors. Publisher and the editors disclaim responsibility for any damage resulting from any ideas, instructions, methods, or products referred to in the content.
 IASGO Society News
IASGO Society News