Surgery, Gastroenterology and Oncology

|
|
Background: Patient reported outcomes are valuable components in the assessment of results of treatment for peripheral arterial disease (PAD). The aim of the study was to translate the six item Vascular Quality of Life Questionnaire (VascuQoL-6) survey into Romanian, and to validate the psychometric performance of the questionnaire in a representative cohort of patients with lower extremity arterial disease.
Material and Methods: Translation of the VascuQoL-6 questionnaire was performed following accepted methodology. The overall validation cohort included 100 patients with PAD (86% with chronic limb-threatening ischemia) undergoing lower limb revascularization. In 20 patients with stable PAD two questionnaires were offered preoperatively with a median interval of 15 days. Another 22 patients were re-tested after revascularization at a median interval of 30 days.
Results: The median time required for completion of the VascuQoL-6 survey was 2 (IQR 2-3) minutes. The translated version demonstrated high internal consistency (Cronbach’s alpha – 0.81) and there was no difference in the preoperative median VascuQoL-6 scoring during the test re-test assessment. Area under the ROC curve for ability to discriminate intermittent claudication from chronic limb-threatening ischemia was 0.897. The median VascuQoL-6 score increased from 10 (IQR 8-12) points preoperatively to 18.5 (IQR 14.7-20) points postoperatively (p < 0.0001) with a standardized response mean of 2.94.
Conclusion: The Romanian version of the VascuQoL-6 survey demonstrated good reliability, validity and responsiveness and can thus be recommended for use in patients with lower limb PAD.
INTRODUCTION
Patient reported outcomes are increasingly recognized as a valuable component in comprehensive assessment of the results of medical and interventional treatment for peripheral arterial disease (PAD). Current clinical practice guidelines on the management of chronic limb ischemia recommend reporting of treatment outcomes based on a combination of objective clinical, anatomical, hemodynamic criteria and evaluation of health-related quality of life (HRQoL) (1). In contrast to a large variety of generic HRQoL questionnaires usually available in multiple linguistic versions, the number of disease-specific tools designed for the cohort of patients with PAD is still limited. The Vascular Quality of Life questionnaire (VascuQoL) questionnaire was developed in 2001 and initially comprised 25 items (2). Nearly a decade later, the short version – the six item VascuQoL questionnaire (VascuQoL-6) was developed and included the six most informative items selected from the original questionnaire based on both classical psychometric methods and item response theory. The VascuQoL-6 demonstrated high validity, reliability and high correlation with the original extended version (3). Since then, VascuQoL-6 has been translated into many languages, and the favorable psychomteric properties have been confirmed by additional validation studies in other countries (Norwegian, Portuguese, Dutch) and there are several ongoing clinical trials registered for validation in other languages (German, Spanish) (4,5). The VascuQoL questionnaire is currently used in the most important randomized clinical trials in patients with chronic limb threatening ischemia: BASIL-2,
BASIL-3 and BEST-CLI (1) and the VascuQoL-6 is being used both in the large registry-based RCT SWEDEPAD that investigate drug-coated devices in PAD (6) and in the phase 3 trial STRIDE on the effect of semaglutide on walking ability in PAD patients (https://clinicaltrials. gov/ct2/show/NCT04560998). To the best of our knowledge no PAD-specific validated HRQoL questionnaires are available in Romanian, hindering the reporting of outcomes and scientific research in the field of vascular surgery at the national level.
The aim of present study was to translate and linguistically validate the VascuQoL-6 questionnaire in Romanian, and to prospectively validate the translated version in a representative cohort of patients with chronic lower limb ischemia.
MATERIAL AND METHODS
This study was conducted in the Vascular Surgery Clinic at the Department of General surgery, „Nicolae Testemitanu” State University of Medicine and Pharmacy from Republic of Moldova. The translation process was performed during January-February 2021 and collection of the data for validation of the translated version was done between June 2021 and February 2022. The authors of VascuQoL-6 (JN, MM) granted permission for translation of the questionnaire. The study was conducted according to the principles outlined in the Declaration of Helsinki and approved by the university’s ethical committee as a component part of the doctoral research project of the second author (PA).
Translation process
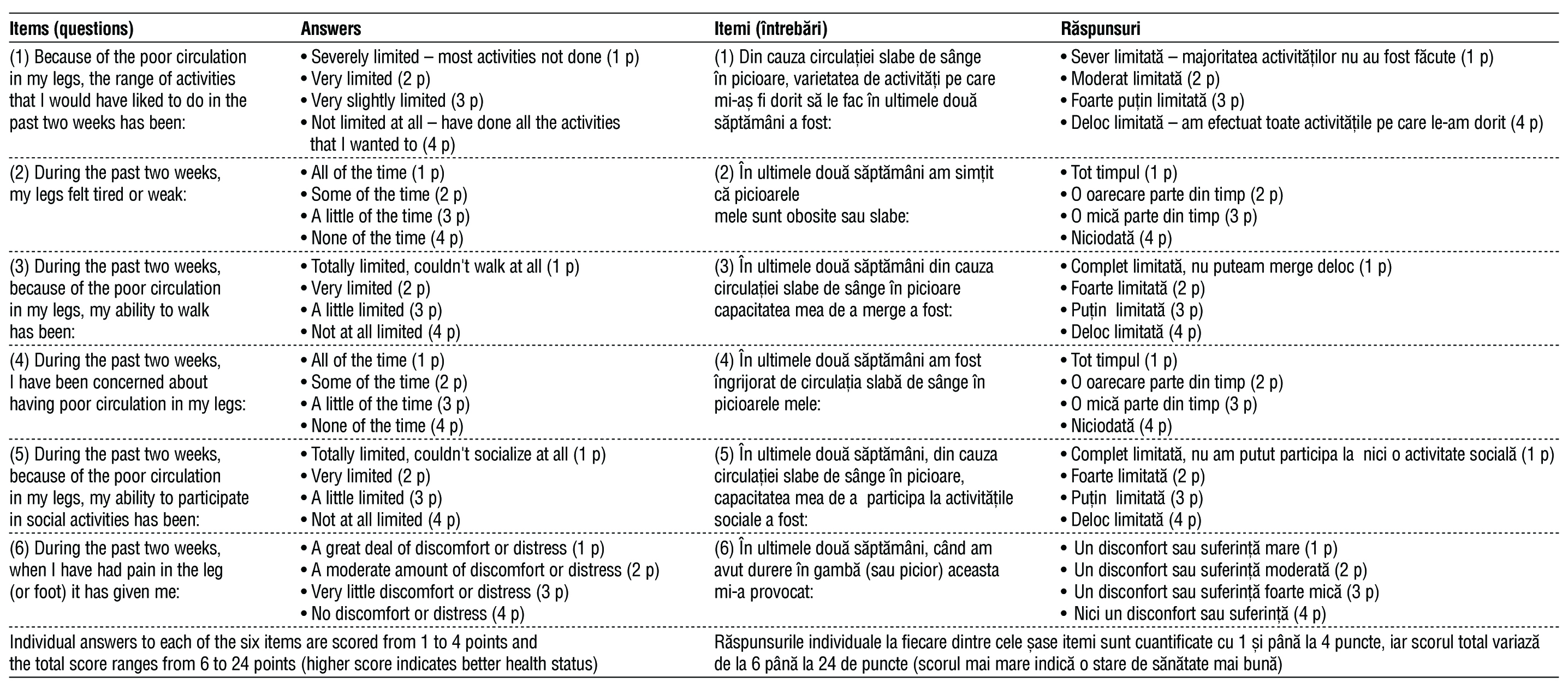
According to current recommendations translation of the VascuQoL-6 questionnaire was done in several consecutive steps (7). Initially, three attending vascular surgeons (native speakers in Romanian and B2 or higher level in English language) independently translated the original version of VascuQoL-6 to Romanian. Next, the same researchers performed the backward translation from Romanian to English exchanging the translated versions between the experts. All translated versions were analyzed by authors of the VascuQoL-6 questionnaire (JN, MM), then discussed as a group until a consensus was reached. At the following stage, three other vascular specialists (not involved in translation process) and five patients with PAD, all – native speakers in Romanian, performed an evaluation of each translated item, answering the following questions: “Is the question formulated clear and easy to understand?”; “Is the question relevant to lower limb ischemia?”; “Should this question be modified?”. Thereafter, the adjustment of the final version of the questionnaire was done and approved by the developers. The original and Romanian versions of “VascuQoL-6” questionnaire are presented in
table 1.
Table 1 - The original (3) and Romanian versions of VascuQoL-6 quality of life questionnaire.
Validation cohort
According to the recommendation provided by Hatcher et al (2014) a minimal sample size of 100
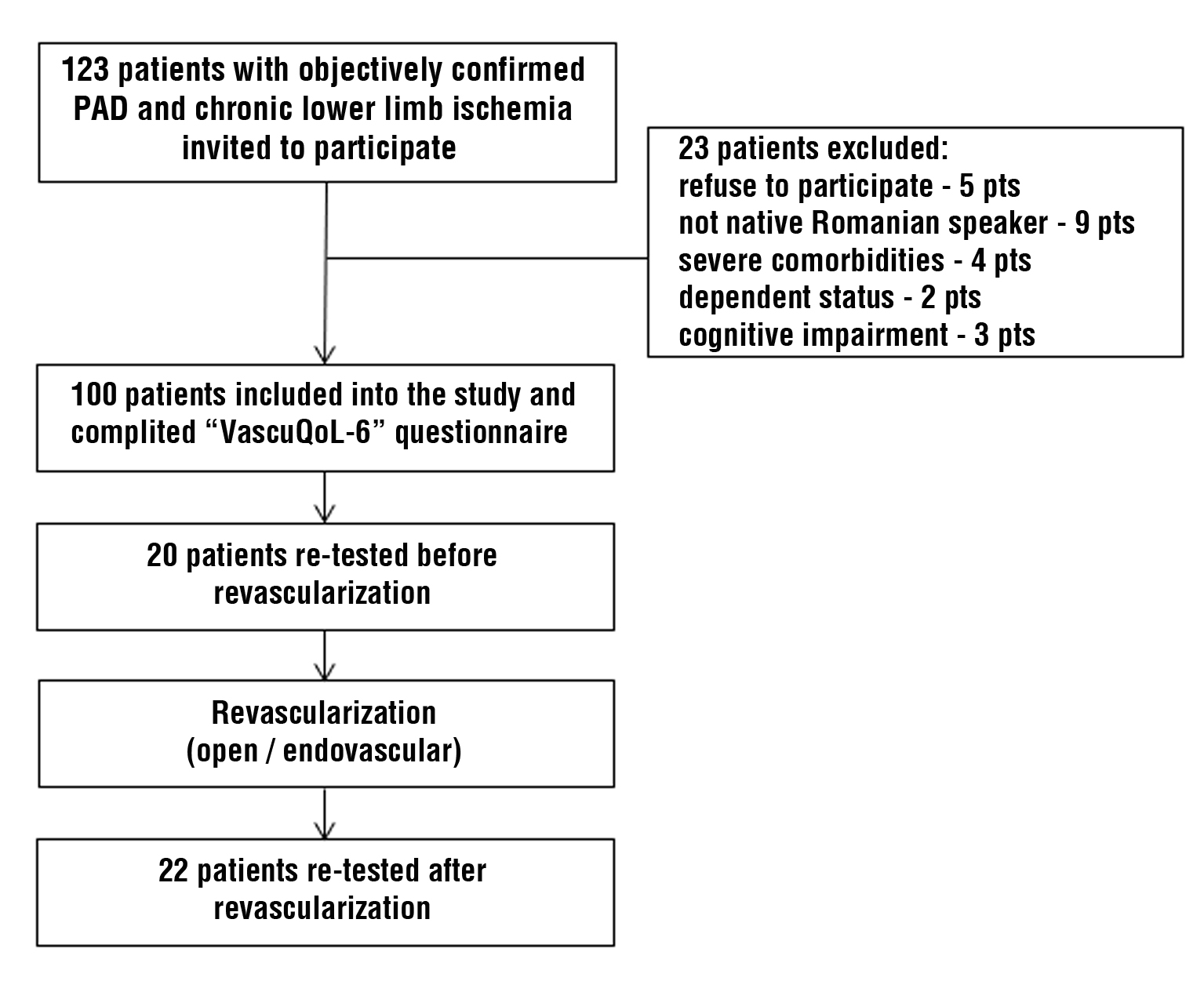
participants was chosen for the validation process (8). During the study period all consecutive patients managed at the institution for objectively confirmed PAD and chronic limb ischemia were invited toparticipate. Twenty-three participants were excluded due to the following reasons: refusal to participate – 5; not a native Romanian speaker – 9; severe comorbidities and/or dependent status – 6; altered mental status or cognitive impairment – 3 patients.
The Romanian version of “VascuQoL-6” was provided to study participants on the day of admission to the Department or during the primary consultation at the outpatient facility. In 20 (20%) patients with stable PAD two questionnaires were offered with an interval between “test” and “re-test” raging from 14 to 30 days (median – 15 days). Another 22 (22%) patients were re-tested following open or endovascular revascularization of the affected lower limb at a median interval of 30 days (range 30 – 90 days). The study flow-chart is presented in fig. 1.
Figure 1 - Study flow-chart demonstrating the inclusion of patients for validation of translated version of VascuQoL-6 questionnaire.
Clinical workup, definitions and classifications
Study participants were required to complete a clinical examination, measurement of the ankle-brachial index (ABI) and vascular imaging (duplex ultrasound, computed tomo-graphy angiography and/or digital subtraction angiography). In patients with intermittent claudication, the self-reported pain-free walking distance was recorded. Sympto-matic lower extremity PAD was defined as presence of typical symptoms of chronic (more than 2 weeks) limb ischemia, abnormal ABI and ≥50% stenosis or occlusion of aorto-iliac, femoro-popliteal or infragenicular arteries. If these criteria were associated with rest pain, presence of plantar ulcer or gangrene, chronic limb threatening ischemia (CLTI) was diagnosed (1). The severity of ischemia was classified by Fontaine stages and the risk of major limb amputation was stratified according to the WIfI (Wound, Ischemia, foot Infection) classification. The mean WIfI score were calculated as proposed by Darling (9). In the 22 patients who completed the VascuQoL-6 questionnaire after revascularization, the corresponding changes in ABI and WIfI stage were registered during the same follow-up visit.
Statistical analysis
The Shapiro-Wilk test was used to test normality of data distribution. Continuous variables are presented as means ± standard deviation or as medians with 25-75% interquartile range (IQR). Categorical variables are presented as number with percentage. Difference of medians was assessed by Mann-Whitney test, and difference between proportions – by Fisher exact test. Correlation coefficient was calculated to measure relationship between variables. A p-value < 0.05 was considered statistically significant. Statistical analysis was performed using „GraphPad Prism” (v. 8.0.1, GraphPad Software, San Diego, California, USA) and SPSS 16.0 (SPSS Inc., Chicago, IL, USA) software.
RESULTS
Patient characteristics and intervention
Mean age of the participants was 67±8 years (range 48-89 years) and 69% were male. Intermittent claudication that restricted daily life activities that was resistant to medical treatment and exercise was diagnosed in 14 (14%) patients, and CLTI in 86: Fontaine stage III – 23 (23%), Fontaine stage IV – 63 (63%) patients. The WIfI stage 1 (very low risk of amputation) was diagnosed in 9 patients, stage 2 (low risk) – in 44, stage 3 (moderate risk) – in 28, and stage 4 (high risk) – in 15 cases (in 4 patients the WIfI stage was not possible to determine due to unreliable ABI values). Open surgical bypass was performed in 54 (54%) patients (11 for aorto-iliac disease and 43 - for infrainguinal disease). Endovascular revascularization (plain balloon angioplasty ± stenting) was used in 46 (46%) cases: aorto-iliac segment – 5, femoro-popliteal and/or infrapopliteal segment – 41.
Psychometric evaluation of the translated VascuQoL-6 questionnaire
All study participants completed the provided questionnaires without asking for assistance or additional explanations. Median time required for completion of the VascuQoL-6 was 2 (IQR 2-3) minutes. Analysis of returned questionaries did not detect any missed items or voluntarily left out responses.
In the entire study cohort, the median value of VascuQoL-6 scored 10 (IQR 8-12) points preoperatively, ranging from 6 to 19 points. The percentage of persons reporting scores below 8 points (< 10% percentile) and above 15 points (> 90% percentile) was 7% and 8%, respectively, demonstrating lack of relevant floor or ceiling effects in the target population. The translated version demonstrated a high reliability with a Cronbach’s alpha coefficient for internal consistency of 0.81. The results of the inter-item correlation matrix assessment varied from 0.24 (Q4 with Q5) to 0.64 (Q1 with Q3).
The analysis of test-retest reliability showed favorable results. Thus, there was no statistical difference between the median VascuQoL-6 score as obtained during the first and second preoperative visit – 13.5 (IQR 9-17) and 13.0 (IQR 8.25-16.75) points, respectively (p > 0.05). The interclass correlation coefficient for the entire questionnaire was 0.86 (95% CI 0.67-0.93), indicating excellent reliability.
The median value of the VascuQoL-6 in patients with intermittent claudication was significantly higher – 15 (IQR 13-18) points, comparing to the patients with CLTI: 10 (IQR 8-12) points in Fontaine stage III and 10 (IQR 8-11) points in Fontaine stage IV, respectively (p < 0.0001). The area under ROC (Receiver Operating Characteristic) curve for the ability of the VascuQoL-6 to discriminate intermittent claudication from CLTI was 0.897, confirming acceptable diagnostic performance of the translated questionnaire (fig. 2). The cut-off value of less than or equal to 12 points offered a sensitivity of 86% and a specificity of 85% in the detection of chronic limb-threatening ischemia. The Romanian version of VascuQoL-6 demonstrated modest but statistically significant correlation with preoperative clinical criteria used for routine stratification of the patients by severity of ischemia. Thus, the correlation coefficient for HRQoL scores was: r = 0.44 (95% CI 0.26-0.59) with the ABI values; r = 0.37 (95% CI 0.18-0.53) with pain free walking distance; and r = -0.36 (95% CI (-0.51) – (-0.17)) with the mean WIfI score.
Figure 2 - The ROC curve for ability of VascuQoL-6 questionnaire to discriminate between patients with intermittent claudication and with limb threatening ischemia.

Technical success of revascularization was accomplished in all CLTI patients that were re-evaluated regarding HRQoL postprocedurally. The mean increase in the ABI value postoperatively was 0.45 ± 0.16,
p < 0.0001. At a median follow-up interval of 30 (IQR 30-60) days the downgrading of WIfI with at least one stage was registered in all patients. The WIfI stage 0 was diagnosed in 6 (27.2%) case, stage 1 – in 15 (68.1%) cases and stage 2 – in one case. The median VascuQoL-6 score increased from 10 (IQR 8-12) points preoperatively to 18.5 (IQR 14.7-20) points postoperatively (p < 0.0001) with higher score in patients categorized postoperatively as WIfI stage 0 vs WIfI stage 1 – 20 (IQR 18.5-22) vs 17 (IQR 13.7-19.2) points, the difference being of borderline statistical significance (p = 0.05). The standardized response mean (mean score difference divided by the standard deviation of the mean score difference) for the VascuQoL-6 questionnaire was 2.94 (Q1 – 1.36, Q2 – 1.46, Q3 – 1.63, Q4 – 1.61, Q5 – 1.09, Q6 – 2.12) indicating a large observable effect size of the invasive intervention by the VascuQoL-6. There was also a moderate positive correlation between the observed ABI increase after intervention and the corresponding change in HRQoL scores: r = 0.45 (95% CI 0.01-0.74).
DISCUSSION
There are several generic and disease-specific QoL questionnaires used for evaluation of patient reported outcomes in peripheral vascular interventions (10). The main advantage of disease-specific tools is their ability to focus on details directly related to the pathological condition and, as a result, an increased sensitivity to modifications in disease severity due to natural progression or after different treatments provided. Comparing to other HRQoL questionaries designed for patients with PAD (“PAQ”, “CLAU-S”, “PAVK-86”) that include from 20 to 86 items, the VascuQoL-6 survey is substantially more practical to use for both patients and practitioners (11-13). Although the median time required for completion of the VascuQoL-6 questionnaire was somewhat longer than that reported by the authors of the original version (1.4 minutes) in the current study (3), it still was highly acceptable for reasonably convenient routine use in clinical practice.
Results of the psychometric evaluation of the Romanian version of VascuQoL-6 were similar to the data reported by other researchers who have translated and validated the questionnaire into otherlanguages. Thus, internal consistency measured by Cronbach’s alpha coefficient was 0.82 for the Norwegian version (4) and 0.84 for the Brazilian-Portuguese version (5). The interclass correlation coefficient of 0.84, determined by de Almeida Correia for test-retest reliability of VascuQoL-6, was almost identical to that presented in current study (5). In contrast to above mentioned studies that included exclusively (4) or predominantly (5) patients with intermittent claudication, CLTI cases represented nearly 90% in our cohort. Despite this fact, thecoefficient of correlation between the total VascuQoL-6 score and pain free walking distance determined in current study (r = 0.37) was similar to that reported for another version – 0.39 (5), emphasizing the usefulness of VascuQoL-6 also in CLTI.
The ability of disease-specific QoL questionnaires to correlate with the results of applied treatments is important. Results in the current study demonstrated appropriate responsiveness of translated version of VascuQoL-6 to invasive revascularization procedures. Successful revascularization thus resulted in significant increase in total VascuQoL-6 score with a mean increase value of 7.4±2.3 points. This observed HRQoL change should also be considered clinically important based on the principle of “more than half of standard deviation” as proposed by Norman (> 1.4 points in our cohort) (14). It should be mentioned however, that the effect size assessed by standardized response mean in current study was even higher than in original version of VascuQoL-6 – 1.15 and in Norwegian version – 1.13 (3,4). We suppose that this difference could be explained by a more pronounced clinical benefit of revascularization in case of CLTI as compared with intermittent claudication.
There are several limitations of our study. The small number of patients treated for intermittent claudication limited the possibilities to analyze the responsiveness of the translated VascuQoL-6 version in this particular subgroup. The results of conservative treatments and impact of failed revascularization attempts upon HRQoL were also not evaluated in the present study which might be considered a limitation and may warrant future study.
CONCLUSION
The translated version of the VascuQoL-6 questionnaire into Romanian was subjected to a comprehensive psychometric evaluation in a representative cohort of patients with lower limb PAD where the majority of patients had CLTI. The characteristics of the Romanian VascuQoL-6: reliability, validity and responsiveness have matched the original version and previous translations of the questionnaire into other languages. Based on this validation study, the VascuQoL-6 questionnaire can thus be recommended for both routine clinical use in patients with PAD as well as for research purposes within the field of vascular surgery.
Authors’ Contributions
Study design (DC, AP, VC); translation of questionnaire and linguistic validation (all authors); data collection and analysis (DC, AP); writing (DC, AP), manuscript revision and final approval (all authors).
Conflicts of interests
MM has copyright to the VascuQoL-25, and MM and JN has copyright to the VascuQoL-6, and must be contacted prior to use. Neither MM or JN received any reimbursement in relation to this study. No other potential conflict of interests is stated by the authors.
Funding: None.
REFERENCES
- Conte MS, Bradbury AW, Kolh P, White JV, Dick F, Fitridge R, et al. Global Vascular Guidelines on the management of chronic limb-threatening ischemia. Eur J Vasc Endovasc Surg. 2019;58(1S):S1-S109.e33.
- Morgan MB, Crayford T, Murrin B, Fraser SC. Developing the Vascular Quality of Life Questionnaire: a new disease-specific quality of life measure for use in lower limb ischemia. J Vasc Surg. 2001;33(4):679-87.
- Nordanstig J, Wann-Hansson C, Karlsson J, Lundström M, Pettersson M, Morgan MB. Vascular Quality of Life Questionnaire - 6 facilitates health-related quality of life assessment in peripheral arterial disease. J Vasc Surg. 2014;59(3):700-7.
- Larsen ASF, Reiersen AT, Jacobsen MB, Kløw NE, Nordanstig J, Morgan M, et al. Validation of the Vascular Quality of Life Questionnaire - 6 for clinical use in patients with lower limb peripheral arterial disease. Health Qual Life Outcomes. 2017;15(1):184.
5. de Almeida Correia M, Andrade-Lima A, Mesquita de Oliveira PL, Domiciano RM, Ribeiro Domingues WJ, Wolosker N, et al. Translation and validation of the Brazilian-Portuguese short version of Vascular Quality of Life Questionnaire in peripheral artery disease patients with intermittent claudication symptoms. Ann Vasc Surg. 2018;51:48-54.
6. Nordanstig J, James S, Andersson M, Andersson M, Danielsson P, Gillgren P, et al. Mortality with Paclitaxel-Coated Devices in Peripheral Artery Disease. N Engl J Med. 2020;383(26):2538-2546.
7. Acquadro C, Conway K, Giroudet C, Mear I. Linguistic validation manual for health outcome assessments. Lyon: MAPI INSTITUTE; 2012.
8. Hatcher L, O'Rourke N. Minimal sample size requirement. In: A step-by-step approach to using the SAS system for factor analysis and structural equation modeling. North Carolina: SAS Institute Inc.; 2014. p. 9.
9. Darling JD, McCallum JC, Soden PA, et al. Predictive ability of the Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification system after first-time lower extremity revascularizations. J Vasc Surg. 2017;65(3):695-704.
10. Hicks CW, Lum YW. Patient-reported outcome measures in vascular surgery. Semin Vasc Surg. 2015;28(2):122-33.
11. Spertus J, Jones P, Poler S, et al. The peripheral artery questionnaire: a new disease-specific health status measure for patients with peripheral arterial disease. Am Heart J. 2004;147:301-8.
12. Finger T, Kirchberger I, Dietze S, et al. Assessing the quality of life of patients with intermittent claudication; psychometric properties of the claudication scale (CLAU-S). Qual Life Res. 1995;4:427.
13. Heidrich H, Bullinger M, Cachovan M, et al. Quality of life in peripheral arterial occlusive disease. Multicenter study of quality of life characteristics with a newly developed disease specific questionnaire. Med Klin (Munich) 1995;90:693-7.
14. Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582-92.
Full Text Sources:
Abstract:
Views: 2377

For Authors
Journal Subscriptions

Sept 2025
Supplements
Instructions for authors
Online submission
Contact
e-ISSN: 2601 - 1700 (online)
ISSN-L: 2559 - 723X
Journal Abbreviation: Surg. Gastroenterol. Oncol.
Surgery, Gastroenterology and Oncology (SGO) is indexed in:
- SCOPUS
- EBSCO
- DOI/Crossref
- Google Scholar
- SCImago
- Harvard Library
- Open Academic Journals Index (OAJI)
Surgery, Gastroenterology and Oncology (SGO) is an open-access, peer-reviewed online journal published by Celsius Publishing House. The journal allows readers to read, download, copy, distribute, print, search, or link to the full text of its articles.
Time to first editorial decision: 25 days
Rejection rate: 61%
CiteScore: 0.2

Meetings and Courses in 2025
Meetings and Courses in 2024
Meetings and Courses in 2023
Meetings and Courses in 2022
Meetings and Courses in 2021
Meetings and Courses in 2020
Meetings and Courses in 2019
Verona expert meeting 2019

Surgery, Gastroenterology and Oncology applies the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits readers to copy and redistribute the material in any medium or format, remix, adapt, build upon the published works non-commercially, and license the derivative works on different terms, provided the original material is properly cited and the use is non-commercial. Please see: https://creativecommons.org/licenses/by-nc/4.0/
Publisher’s Note:
The opinions, statements, and data contained in article are solely those of the authors and not of Surgery, Gastroenterology and Oncology journal or the editors. Publisher and the editors disclaim responsibility for any damage resulting from any ideas, instructions, methods, or products referred to in the content.
 IASGO Society News
IASGO Society News