Surgery, Gastroenterology and Oncology

|
|
Gastric carcinomatosis is an ominous disease, with a survival of 3-6 months. In the last decade, cytoreduction surgery and hyperthermic chemotherapy by laparotomy have made progress in the treatment of carcinomatosis. Laparoscopic surgery has shown its benefits in the treatment of colon and gastric cancer, obesity, with lower rates of morbidity, shorter hospital stays, and early return to cancer treatment. There are few studies on the use of laparoscopy in gastric carcinomatosis, Arjona et al. (2021,2023) put together an international registry of laparoscopy and carcinomatosis, which included one case of gastric carcinomatosis out of 315 patients operated by L-CRS-HIPEC. Our objective is to show a simplified laparoscopic technique, the simplified HIPEC method (simplified LS-CRS-HIPEC), not yet reported in the literature, for the treatment of gastric carcinomatosis and to show the early clinical results of the technique in one patient. The case of a 44-year-old man with diffuse antral gastric adenocarcinoma and laparoscopic peritoneal carcinomatosis index (PCI) 4. Carcinomatosis managed in our department using simplified L-CRS-HIPEC in Cali, Colombia, is presented. The patient received neoadjuvant treatment followed by D2-laparoscopic total gastrectomy and L-HIPEC. The procedure time was 240 minutes and the intraperitoneal temperature was 42°C for 60 minutes. There were no postoperative complications or death. The hospital stay was 6 days, with 1 day in the ICU. The return to adjuvant time was 4 weeks and patient is eight- months disease free. Simplified L-CRS-HIPEC is a a safe surgical method for gastric carcinomatosis that decrease the surgical time and the time of return to oncological
treatment.
INTRODUCTION
Gastric carcinomatosis is an ominous disease, with a survival of 3-6 months. In the last decade, cytoreduction surgery and hyperthermic chemotherapy by laparotomy have made advances in the treatment of carcinomatosis.
Laparoscopic surgery has manifest benefits in different tumors: colon, stomach, with lower rates of morbidity, less bleeding, shorter hospital stays, and earlier return to cancer treatment. Similar benefits of laparoscopic surgery in obesity have been reported, with simplified techniques that decrease the rates of complications (1). Studieson the use of laparoscopic surgery for gastric carcinomatosis are scarce (2,3). Balescu (2017) described the L-CRS laparoscopic surgical technique for two patients operated on without morbidity or mortality (4).
Our objective is to show a simplified laparoscopic technique, the simplified HIPEC method (simplified LS-CRS HIPEC), not yet reported in the literature, for the treatment of gastric carcinomatosis and to report the early clinical results of the technique in one patient.
Case Report
The patient reported here signed the required informed consents. The case of a 44-year-old man with diffuse antropyloric gastric adenocarcinoma T4aN1M1 EIV is presented according to the 8th edition of the staging system of the American Joint Committee on Cancer. He had a Peritoneal Carcinomatosis Index (PCI) of 4 by laparoscopy and positive peritoneal biopsies for metastatic gastric adenocarcinoma. Human epidermal growth factor receptor 2 (HER2): negative. Programmed cell death receptor ligand 1 (PDL1): negative. Immunoscore: combined positive score (CPS) score of 0. Negative chest tomography for metastases. Abdominal tomography showed concentric thickening of the pylorus to the serosa and perigastric nodes up to 1.2 cm. Upper gastrointestinal endoscopy: ulcerated lesion of 28 mm, with fibrin that deformed the pylorus and caused obstruction of 50% with pyloric syndrome. Loss of 20 kg in 2 months. Weight and Karnofsky index (KI) at admission: 52 kg and 90%. He was hospitalized urgently for total parenteral nutrition supplementation and initiation of neoadjuvant chemotherapy with the FLOT4 regimen. After the first cycle of chemotherapy, the patient began to tolerate a soft and normal diet. He was discharged at 2 weeks to continue neoadjuvant and prehabilitation with physical therapy, muscular strengthening by resistance training twice a week, and three walks a week totaling 150 minutes, following the recommendations given by the World Health Organization. At week 12 after chemotherapy, after resolution of respiratory symptoms and 3-week disability in the period of the COVID-19 pandemic, he regained 18 kg and had KI = 100%. Radical resection was performed with the laparoscopic simplified method L-CRS-HIPEC, at the Sebastián de Belalcázar Clinic in the city of Santiago de Cali, Colombia.
SURGICAL TECHNIQUE
The simplified surgical method for gastric carcinomatosis (simplified LS-CRS HIPEC) procedure was as follows:
The patient was put into the Lloyd-Davies position (5). Total laparoscopic gastrectomy and laparoscopic D2 abdominal lymphadenectomy were done as described by Muguruma et al. (6). Cytoreduction and laparoscopic hyperthermic chemotherapy (L-CRS-HIPEC) and anesthetic techniques were as described by Balescu et al. (4). Laparoscopic cholecystectomy was done as described by Vishal Gupta and Gaurav J. (7). We applied the following particular modifications of the simplified HIPEC method (simplified LS-CRS HIPEC): Two 5-mm accessory trocars were introduced for right peritonectomies, one on the left midclavicular line 4 cm supraumbilical, and the other 2.5 cm medial to the left superior anterior iliac spine. We used the same procedure for inserting two 5-mm trocars on the right side for left peritonectomies. The procedure began with low-volume laparoscopic peritonectomies, making a small incision at 1 cm around the peritoneal metastasis and with Maryland forceps, creating a subperitoneal tunnel and allowing the entry and dissection of the peritoneum with CO2 for later removal of the dissected area with bipolar forceps. An advanced surgical specimen extraction for labeling can be seen in fig. 1.
Figure 1 - Creation of a subperitoneal tunnel that allowed entry and dissection of the peritoneum with CO2 for later removal of the dissected area with bipolar forceps.
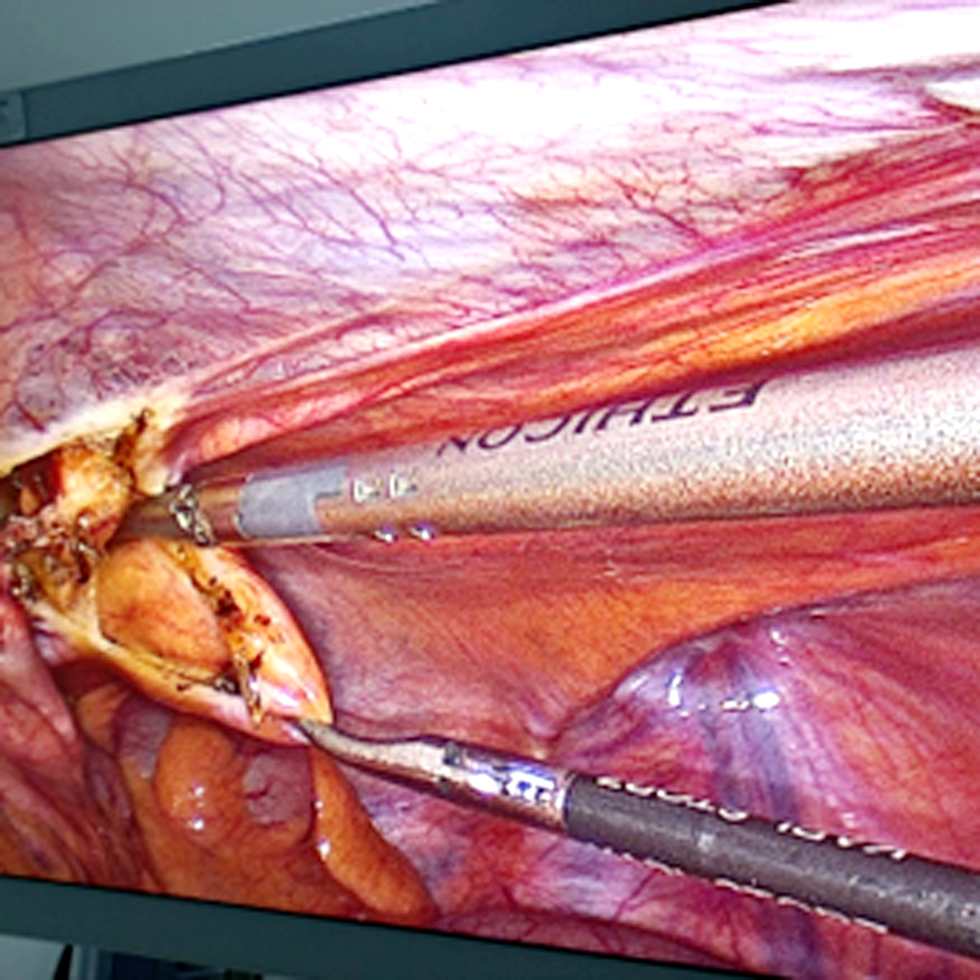
After performing laparoscopic total gastrectomy and laparoscopic D2 abdominal lymphadenectomy fig. 2, laparoscopic hipec (L-HIPEC) was performed as described by Balescu et al (4). The phrenoesophageal ligaments were released and the phrenic nerves were sectioned with advanced bipolar forceps until an esophagus of approximately 7 cm was obtained in the abdominal cavity. We sectioned the esophagus to 85% of its circumference at 2 cm from the gastroesophageal junction with a 45-mm purple stapler to allow the stomach to pull the esophagus from the mediastinum to the abdominal cavity. Next were identification of the angle of Treitz, follow-up and elevation of the jejunal loop for esophageal reconstruction, sectioning of the jejunal loop 50 cm from the Treitz with a 45-mm sand-colored Signia stapler, marking of the biliary loop with a titanium clip, and use of a grasper for jejuno-jejunal reconstruction.
Figure 2 - D2-lymphadenectomy and sectioning left gastric artery with stapler.
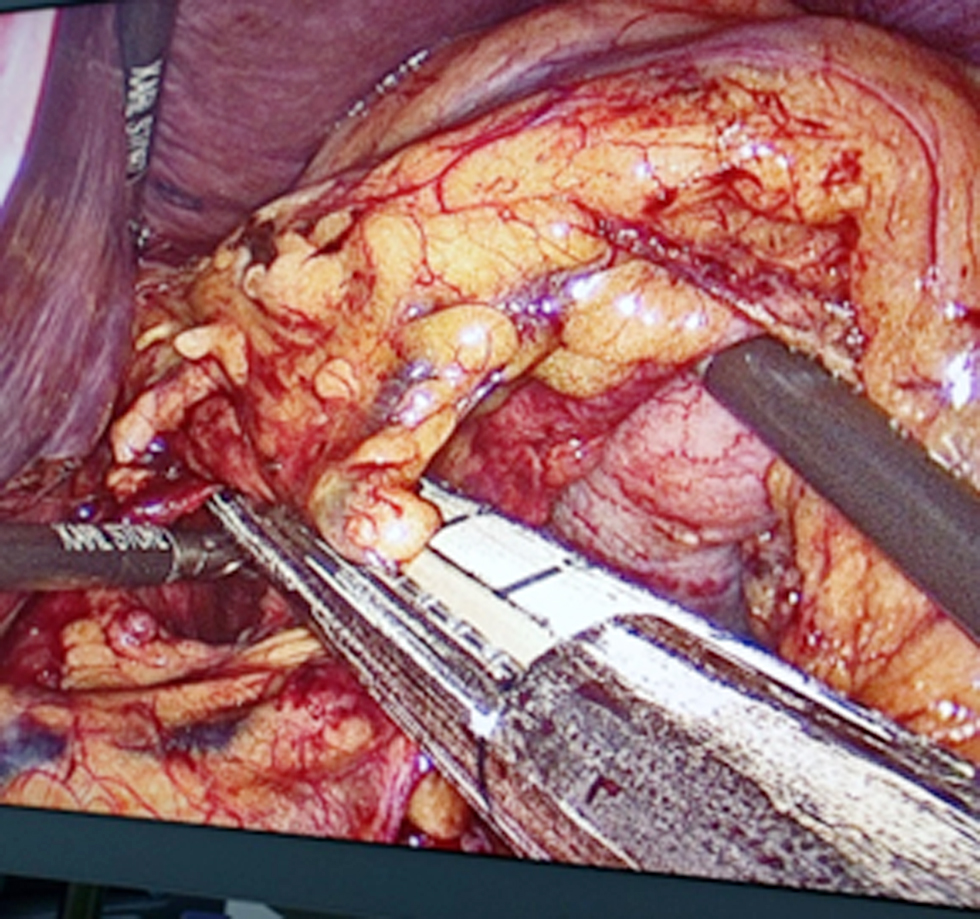
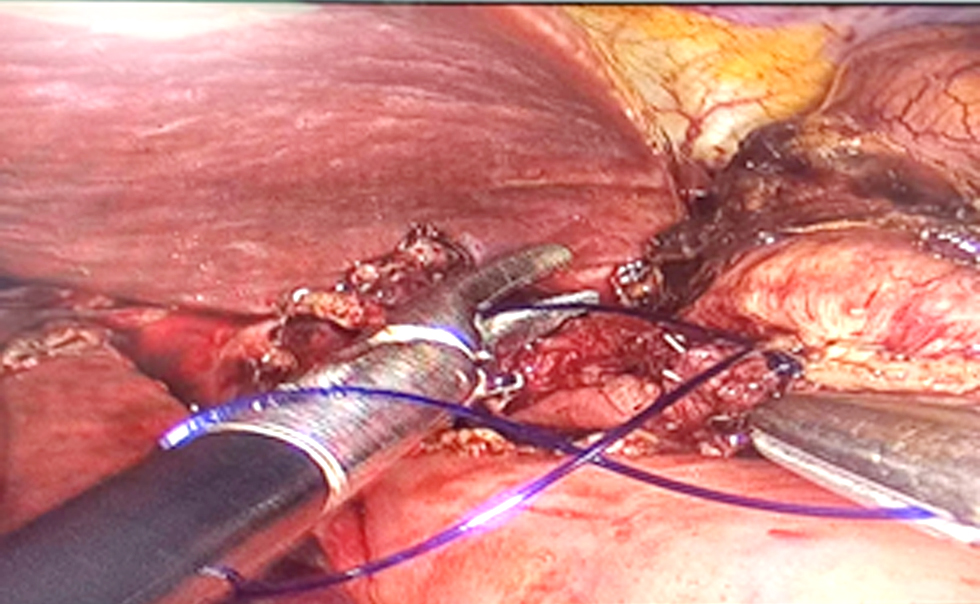
We performed laterolateral esophagojejunal anastomosis and reconstruction with a linear stapler, esophagostomy smaller than 1 cm in diameter with the help of an 18-mm orogastric tube, and identification of the esophageal mucosa and the hole for Esophago-Yeyuno anastomosis. Next were the ascent of the previously sectioned jejunal loop and performance of an enterotomy of less than 1 cm with advanced bipolar, use of a 45-mm purple stapler on the previously made esophageal and jejunal holes, removal of the stapler, and manual suture of the esophagus jejunum anastomosis with PDS 3-0 Equidistant with reinforcement points at three sites with 3-0 PDS (fig. 3). A leak test was done with methylene blue. Complete laparoscopic anastomosis reconstruction was assisted with an Alexis M separator through a 5-cm supraumbilical incision made with a 45-mm Signia stapler, and the enterotomy was closed with PDS 3-0. We introduced fenestrated hyperthermia cannulas and abdominal thermometers into the abdominal cavity through the 5-cm supraumbilical incision with a Medtronic kit, oriented the entry and exit cannulas toward the parietocolic s, fixed them with 1-0 silk to the fascia, made a temporary skin suture with 1-0 prolene, performed functional verification of the system connected to the HT 1000 hyperthermia machine, introduced 3 liters of sodium chloride at a temperature of 39 °C for 5 min, raised the temperature to 42 °C for 1 hour of infusion, and introduced chemotherapy with mitomycin 20 mg in a total of 4 liters of sodium chloride from the system. Anesthesiologists and perfusionists verified the temperature and hemodynamic variables. Cytoreduction time: L-CRS 180 minutes; HIPEC time 1 hour; total surgical time 4 hours.
Figure 3 - Laterolateral esophagojejunal anastomosis and reconstruction with a linear stapler and manual suture.
Optimal oncological cytoreduction (CCR0): The patient bled 150 ml. He stayed in the intensive care unit 1 day and was hospitalized 6 days. There were no complications, and there was no need to transfuse the patient. Clinical follow-up was continued with acceptable postoperative evolution, chemotherapy was restarted at 4 weeks postoperatively and patient is 8-months disease free.
Discussion
The prognosis of gastric cancer is poor in most regions of the world (8) and is even higher with carcinomatosis. In the last decade, cytoreduction surgery and hyperthermic chemotherapy by laparotomy have made progress in the treatment of carcinomatosis (5-8). The benefits of laparoscopic surgery have been demonstrated in the treatment of different tumors: colon, (13-15) stomach, (16-18) ovary, low-grade peritoneal pseudomyxoma, multicystic mesothelioma, and carcinomatosis, (2,3,16) with low rates of morbidity, short hospital stays, and early return to cancer treatment. Similar benefits of laparoscopic surgery in obesity have been reported, and with simplified techniques that decrease the rates of complications and allow easy reproducibility for teaching (1,17,18), our work is the first description of a simplified technique for the treatment of gastric carcinomatosis. Studies of the extra benefit of laparoscopic surgery in gastric carcinomatosis are scarce. Fourteen international hospitals contributed cases to the PSOGI international registry, laparoscopy being the most frequently used for low-grade peritoneal pseudomyxoma carcinomatosis (55%), benign multicystic meso-thelioma (14%), colon cancer (12.5%), and ovary cancer (9%) and being recommend in low-volume carcinomatosis of grade PCI 3 (2-5) and nonaggressive histopathologies (2,3). Balescu (2017) described in detail the L-CRS laparoscopic surgical technique for two patients operated on without morbidity or mortality in Romania, considering that L-CRS-HIPEC in low-volume gastric carcinomatosis was successfully performed with very good clinical results and that the patients simultaneously received the benefits of both laparoscopy and cytoreduction (4). Gastric cancer is one of the pathologies with the highest incidence and mortality in Colombia. Given the relatively young age of our patient, his KI of 100%, and PCI grade of 4, and the experience of the main surgeon (JPF) and the surgical group in performing laparoscopic oncological procedures, we designed (JPF) and applied this novel simplified surgical technique for the treatment of gastric carcinomatosis. Our case is one of the few published reports in the Western world of performing LS-CRS-HIPEC for gastric carcinomatosis.
Conclusion
The simplified L-CRS L-HIPEC surgical method for gastric carcinomatosis has proven to be a safe surgical method that decreases the surgical time and the time of return to oncological treatment. One limitation was the sample size.
Conflict of interest
The authors declare no conflict of interests.
Ethical statement
Ethical approval was obtained.
References
1. Ramos AC, Silva AC, Ramos MG, Canseco EG, Neto G, de Almeida Menezes M, et al. Simplified gastric bypass: 13 years of experience and 12,000 patients operated. Arq Bras Cir Dig. 2014;27 Suppl 1(Suppl 1):2-8. English, Portuguese
2. Arjona-Sanchez A, Aziz O, Passot G, Salti G, Esquivel J, Van der Speeten K, et al. (2021) Laparoscopic cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for limited peritoneal metastasis. The PSOGI international collaborative registry. Eur J Surg Oncol. 2021;47(6):1420-1426.
3. Arjona-Sanchez A, Aziz O, Passot G, Salti G, Serrano A, Esquivel J, et al. (2023) Laparoscopic cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: Long term oncologic outcomes from the international PSOGI registry. Eur J Surg Oncol. 2023;49(10): 107001.
4. Balescu I, Godoroja D, Gongu M, Tomulescu V, Copaescu C. Laparoscopic HIPEC for Peritoneal Carcinomatosis from Gastric Cancer - Technique and Early Outcomes of Our First Cases. Chirurgia (Bucur). 2017;112(6):714-725.
5. Lloyd-Davies OV. Advantages of the lithotomy-Trendelenburg position in the excision of carcinoma of the rectum. Proc R Soc Med. 1959;52(Suppl)(Suppl 1):40-1.
6. Muguruma K, Tanaka H, Sakurai K, Toyokawa T, Kubo N, Yamashita Y, et al. Laparoscopy-assited total gastrectomy: A simplified Approach. Int Surg. 2014;99(1):79-85.
7. Vishal Gupta, Gaurav J. Safe laparoscopic cholecystectomy: Adoption of universal culture of safety in cholecystectomy. World J Gastrointest Surg. 2019;11(2):62-84.
8. Morgan E, Arnold M, Camargo MC, Gini A, Kunzmann AT, Matsuda T, et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020-40: A population-based modelling study. EClinicalMedicine. 2022;47:101404.
9. Sugarbaker PH, Jablonski KA. Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann Surg. 1995;221(2):124-32.
10. Yonemura Y, Kawamura T, Bandou E, Takahashi S, Sawa T, Matsuki N. Treatment of peritoneal dissemination from gastric cancer by peritonectomy and chemohyperthermic peritoneal perfusion. Br J Surg. 2005;92(3):370-5.
11. Yonemura Y, Ishibashi H, Mizumoto A, Fujita T, Liu Y, Wakama S, et al. Rationale of Treatment for Peritoneal Metastasis. Gan to kagaku ryoho. Gan To Kagaku Ryoho. 2022;49(13):1723-1726.
12. Fujimoto S, Takahashi M, Mutou T, Kobayashi K, Toyosawa T. Successful intraperitoneal hyperthermic chemoperfusion for the prevention of postoperative peritoneal recurrence in patients with advanced gastric carcinoma. Cancer. 1999;85(3):529-34.
13. Deijen CL, Vasmel JE, de Lange-de Klerk ESM, Cuesta MA, Coene PLO, Lange JF, et al. (2017) Ten-year outcomes of a randomised trial of laparoscopic versus open surgery for colon cancer. Surg Endosc. 2017;31(6):2607-2615.
14. Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Jaap Bonjer H, et al. (2005) Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6(7):477-84.
15. Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AMH, et al. (2005) Short-term endpoints of conventional versus laparos-copic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365(9472):1718-26.
16. Kim W, Kim HH, Han SU, Kim MC, Hyung WJ, Ryu SW, et al. (2016) Decreased Morbidity of Laparoscopic Distal Gastrectomy Compared with Open Distal Gastrectomy for Stage I Gastric Cancer: Short-term Outcomes from a Multicenter Randomized Controlled Trial (KLASS-01). Ann Surg. 2016;263(1):28-35.
17. Hyung WJ, Yang HK, Han SU, Lee YJ, Park JM, Kim JJ, et al. A feasibility study of laparoscopic total gastrectomy for clinical stage I gastric cancer: a prospective multi-center phase II clinical trial, KLASS 03. Gastric Cancer. 2019;22(1):214-222.
18. Takeshita K, Liu Y, Ishibashi H, Yonemura Y. Laparoscopic Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Carcinomatosis from Gastric Cancer: Its Beneficial Effects on Reduction and Exact Evaluation of the Peritoneal Cancer Index. Am Surg. 2017;83(11):1315-1320.
Full Text Sources:
Abstract:
Views: 1084

For Authors
Journal Subscriptions

Jun 2025
Supplements
Instructions for authors
Online submission
Contact
e-ISSN: 2601 - 1700 (online)
ISSN-L: 2559 - 723X
Journal Abbreviation: Surg. Gastroenterol. Oncol.
Surgery, Gastroenterology and Oncology (SGO) is indexed in:
- SCOPUS
- EBSCO
- DOI/Crossref
- Google Scholar
- SCImago
- Harvard Library
- Open Academic Journals Index (OAJI)
Surgery, Gastroenterology and Oncology (SGO) is an open-access, peer-reviewed online journal published by Celsius Publishing House. The journal allows readers to read, download, copy, distribute, print, search, or link to the full text of its articles.
Time to first editorial decision: 25 days
Rejection rate: 61%
CiteScore: 0.2

Meetings and Courses in 2025
Meetings and Courses in 2024
Meetings and Courses in 2023
Meetings and Courses in 2022
Meetings and Courses in 2021
Meetings and Courses in 2020
Meetings and Courses in 2019
Verona expert meeting 2019

Surgery, Gastroenterology and Oncology applies the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits readers to copy and redistribute the material in any medium or format, remix, adapt, build upon the published works non-commercially, and license the derivative works on different terms, provided the original material is properly cited and the use is non-commercial. Please see: https://creativecommons.org/licenses/by-nc/4.0/
Publisher’s Note:
The opinions, statements, and data contained in article are solely those of the authors and not of Surgery, Gastroenterology and Oncology journal or the editors. Publisher and the editors disclaim responsibility for any damage resulting from any ideas, instructions, methods, or products referred to in the content.
 IASGO Society News
IASGO Society News