Surgery, Gastroenterology and Oncology
|
|
ABSTRACT
Postoperative recurrence of cancer in patients with p-stage I rectal cancer is rare. Lung or liver metastases from colorectal cancer have been commonly reported. We observed an extremely rare metastasis, namely, vaginal metastasis, from p-stage I rectal cancer one year after surgery. An 82-year-old female patient underwent laparoscopic high anterior resection for p-stage I upper rectal cancer. She received no adjuvant chemotherapy after surgery. Preoperative positron emission tomography combined with computed tomography (PET/CT) revealed no distant metastasis. However, multiple lung metastases and a vaginal metastasis were detected by PET/CT one year after surgery. The biopsy from the vaginal metastasis revealed a well-differentiated adenocarcinoma that was similar to the primary rectal carcinoma. Since rare metastatic recurrence, such as vaginal metastasis, cannot be detected by routine examinations, including CT, PET/CT is very useful for detecting rare metastases.
INTRODUCTION
Postoperative recurrence of cancer in patients with pathological stage I rectal cancer is rare. Furthermore, vaginal metastasis from colorectal cancer is extremely rare. We observed vaginal metastasis from pathological stage I rectal cancer one year after surgery.
CASE REPORT
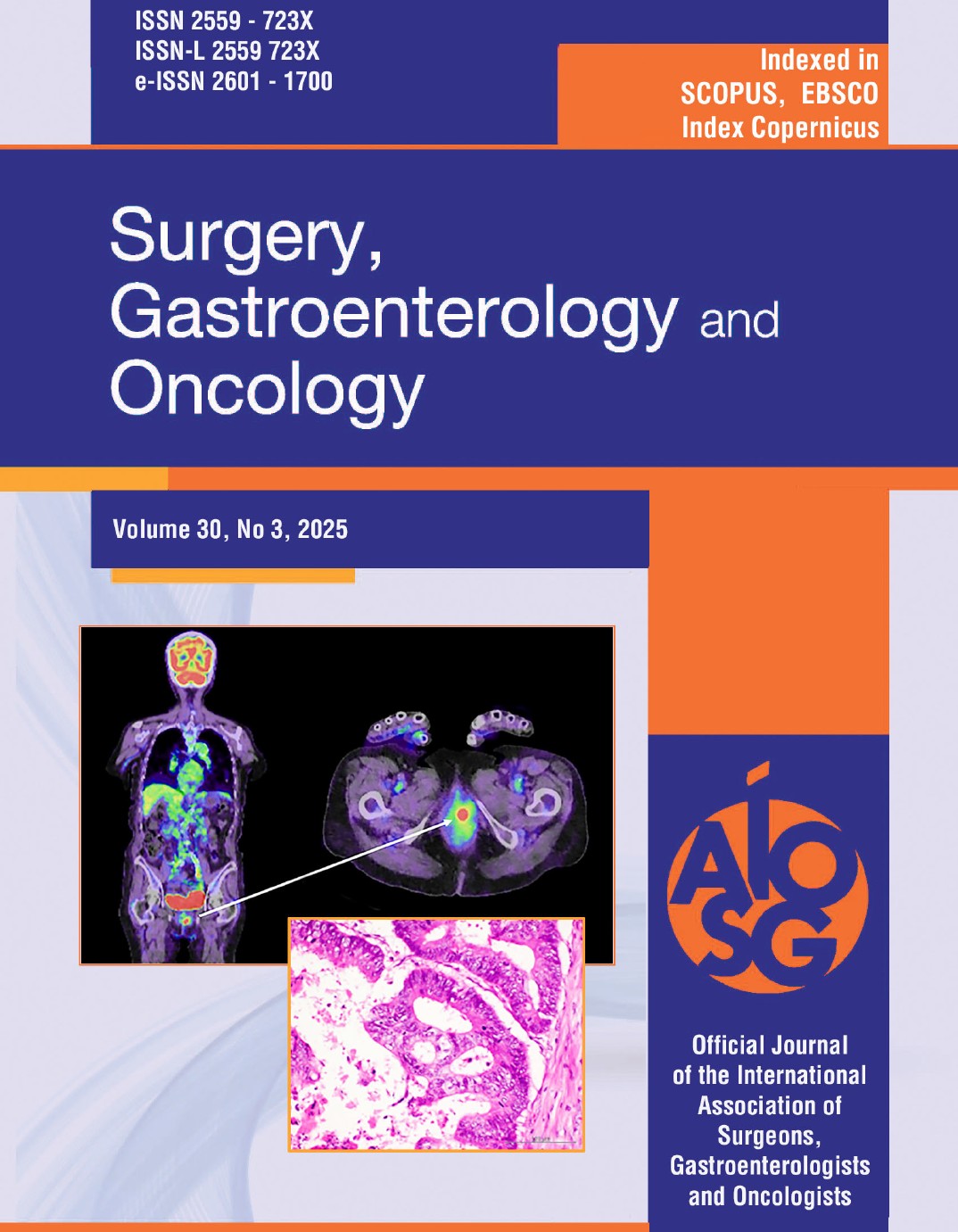
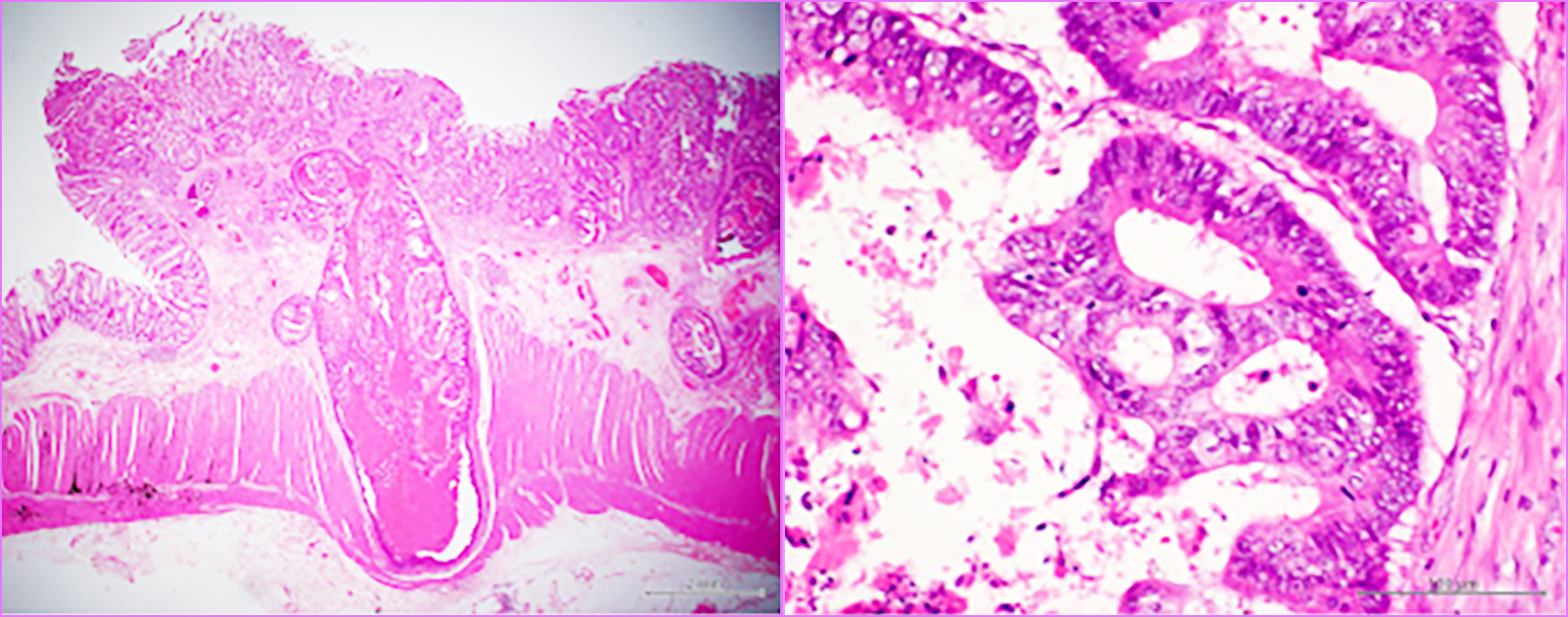
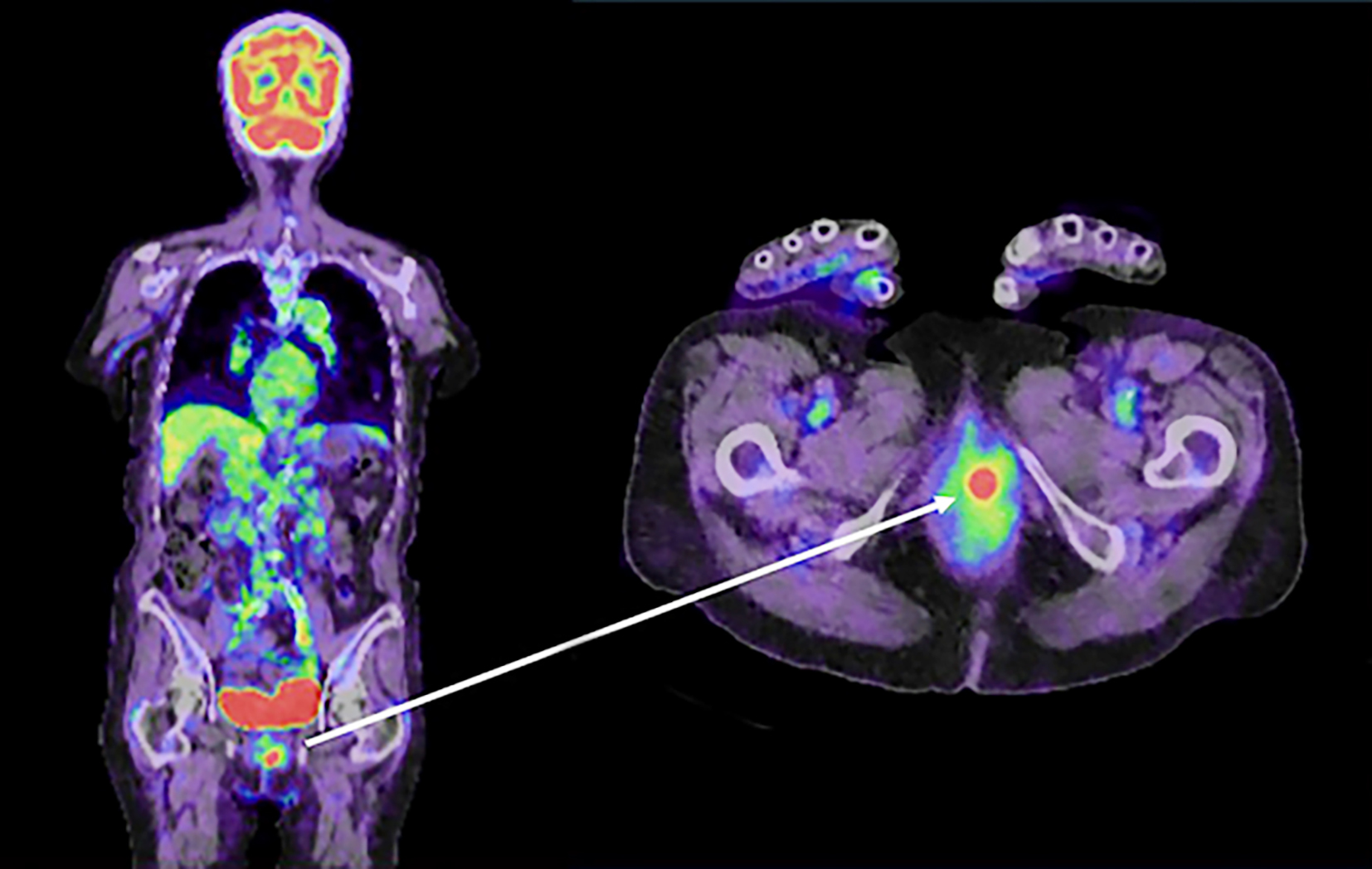
An 82-year-old female patient underwent laparoscopic high anterior resection for pathological stage I upper rectal cancer. Pathological examination of the resected specimen revealed a well-differentiated adenocarcinoma with a diameter of 30 mm, a T2 type-2 tumor, no lymphatic invasion (ly0), venous invasion (v1), and no lymph node metastases (n0) (fig. 1). Laboratory data before surgery revealed an elevated carcinoembryonic antigen (CEA) level (14.6 ng/ml). However, the CEA level normalized after surgery. Preoperative positron emission tomography combined with computed tomography (PET/CT) revealed no distant metastasis. Because the patient was over eighty years old and was diagnosed with early-stage rectal cancer with no distant metastasis, she received no adjuvant chemotherapy after surgery. Laboratory data one year after surgery revealed an elevated CEA level of 17.0 ng/ml, and multiple lung metastases were detected by CT. PET/CT one year after surgery revealed multiple lung metastases and a vaginal tumor (fig. 2). The vaginal tumor was detected on the left side of the vagina by the naked eye. The biopsy from the vaginal tumor revealed a well-differentiated adenocarcinoma, which was similar to the primary rectal carcinoma (fig. 3). The patient started receiving intensive chemo-therapy, including FOLFOX, immediately after the diagnosis. However, she died due to multiple lung metastases one year after chemotherapy was initiated.
Figure 1 - Pathological examination of the primary resected specimen.
T2 tumor, no lymphatic invasion, and venous invasion. (b) A well-differentiated adenocarcinoma.
Figure 2 - PET/CT one year after surgery revealed vaginal metastasis.
Accumulation, which was suspected to be metastasis, was detected in the vagina
Figure 3 - Macroscopic and pathological examination of the vaginal lesion.
(a) The tumor was located on the left side of the vagina. (b) Pathological examination revealed that the vaginal tumor was a well-differentiated adenocarcinoma that was similar to the primary rectal carcinoma.
DISCUSSION
The Surveillance, Epidemiology and End Results Program (SEER) reports an excellent prognosis, with greater than 90% 5-year survival in patients with stage I colorectal cancer (1). The Japanese Society for Cancer of the Colon and Rectum (JSCCR) reports recurrence rates of 4.0% in patients with pT1 disease and 7.3% in patients with pT2 disease (2). A pT2 stage tumor (3-5), lymphovascular invasion (3,6), an infiltrative growth pattern (7), lower rectal lesions (4), male sex (6), a poorly differentiated carcinoma (6) and a high pre-operative CEA value (5) were identified as influential factors related to the recurrence of stage I disease. Only two of the seven items, pT2 stage tumor and high preoperative CEA value, were associated with the present patient. In recent years, BRAF mutation has attracted attention as a risk factor for early recurrence of colorectal cancer after surgery (8,9). The present patient was found to have a BRAF mutation by pathological examination of the primary rectal carcinoma.
The present patient had multiple lung metastases and a vaginal metastasis. The pathological type of primary vaginal carcinoma is most commonly squamous cell carcinoma. Other pathological types, such as adenocarcinoma, are extremely rare. When a vaginal tumor is pathologically detected as an adenocarcinoma, we must consider the location of the primary tumor. The primary tumor associated with metastatic vaginal adenocarcinoma is most commonly located in the uterus and very rarely in the colon, rectum, kidney, breast, or pancreas (10). All patients with vaginal metastasis from rectal carcinoma have been diagnosed with advanced rectal carcinoma greater than stage III (11). There have been no reports of vaginal metastasis from stage I rectal carcinoma.
Mechanisms leading to the formation of vaginal metastases generally involve three pathways: lymphovascular pathways, direct infiltration through the pouch of Douglas, and pathways involving the fallopian tubes (12). Because the patient’s rectal tumor had no extramural extension and no lymph node metastases, involvement of the lymphovascular pathway was highly suspected.
In the present patient, vaginal metastasis could not be detected by routine examinations, including CT. However, the lesion was incidentally detected by PET/CT. The reason for vaginal metastasis from rectal carcinoma to be considered a rare entity may be that routine examination cannot detect it. PET/CT is very useful for detecting rare distant metastases. To the best of our knowledge, this is the first report of vaginal metastasis from stage I rectal cancer one year after surgery.
CONCLUSION
Since rare metastatic recurrence, such as vaginal metastasis, cannot be detected by CT, PET/CT is very useful for detecting rare metastases.
Authors’ Contributions
All authors contributed acquisition of data, analysis and interpretation of data, drafting of manuscript, critical revision of manuscript, and final approval of the version to be published.
Conflict of Interest
The authors declare no financial conflict of interest.
Ethics Approval
This research was conducted ethically in accordance.
REFERENCES
1. Edge SB; American Joint Committee on Cancer. AJCC cancer staging manual. 7th ed. New York: Springer; 2010.
2. Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, et al. Japanese society for cancer of the colon and rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25(1):1-42.
3. Willett CG, Lewandrowski K, Donnelly S, Shellito PC, Convery K, Eliseo R, et al. Are there patients with stage I rectal carcinoma at risk for failure after abdominoperineal resection? Cancer 1992;69: 1651-5.
4. Sticca RP, Rodriguez-Bigas M, Penetrante RB, Petrelli NJ. Curative resection for stage I rectal cancer: natural history, prognostic factors, and recurrence patterns. Cancer Invest. 1996;14(5):491-7.
5. Wichmann MW, Müller C, Hornung HM, Lau-Werner U, Schildberg FW; Colorectal Cancer Study Group. Results of long-term follow-up after curative resection of Dukes A colorectal cancer. World J Surg. 2002;26(6):732-6.
6. Blumberg D, Paty PB, Picon AI, Guillem JG, Klimstra DS, Minsky BD, et al. Stage I rectal cancer: identification of high-risk patients. J Am Coll Surg. 1998;186(5):574-9; discussion 579-80
7. Keum MA, Lim SB, Kim SA, Yoon YS, Kim CW, Yu CS, et al. Clinicopathologic factors affecting recurrence after curative surgery for stage I colorectal cancer. J Korean Soc Coloproctol. 2012; 28(1):49-55.
8. Jin Z, Zou Q, Zhou T, Xue T. Preoperative prediction of early recurrence in patients with BRAF mutant colorectal cancer using a intergrated nomogram. Sci Rep. 2024;14(1):25320.
9. Pappas L, Quintanilha JCF, Huang RSP, Parikh AR. Genomic alterations associated with early-stage disease and early recurrence in patients with colorectal cancer. Oncologist. 2025;30(2):oyae269.
10. Ng HJ, Aly EH. Vaginal metastases from colorectal cancer, Int J Surg. 2013;11(10):1048-55.
11. Sadatomo A, Koinuma K, Horie H, Lefor AK, Sata N. An isolated vaginal metastasis from rectal cancer. Ann Med Surg (Lond). 2015; 5:19-22.
12. Marchal F, Leroux A, Hoffstetter S, Granger P. Vaginal metastasis revealing colon adenocarcinoma. Int J Colorectal Dis. 2006; 21(8):861-2.
Full Text Sources:
Abstract:
Views: 7

For Authors
Journal Subscriptions

Sept 2025
Supplements
Instructions for authors
Online submission
Contact
e-ISSN: 2601 - 1700 (online)
ISSN-L: 2559 - 723X
Journal Abbreviation: Surg. Gastroenterol. Oncol.
Surgery, Gastroenterology and Oncology (SGO) is indexed in:
- SCOPUS
- EBSCO
- DOI/Crossref
- Google Scholar
- SCImago
- Harvard Library
- Open Academic Journals Index (OAJI)
Surgery, Gastroenterology and Oncology (SGO) is an open-access, peer-reviewed online journal published by Celsius Publishing House. The journal allows readers to read, download, copy, distribute, print, search, or link to the full text of its articles.
Time to first editorial decision: 25 days
Rejection rate: 61%
CiteScore: 0.2

Meetings and Courses in 2025
Meetings and Courses in 2024
Meetings and Courses in 2023
Meetings and Courses in 2022
Meetings and Courses in 2021
Meetings and Courses in 2020
Meetings and Courses in 2019
Verona expert meeting 2019

Surgery, Gastroenterology and Oncology applies the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits readers to copy and redistribute the material in any medium or format, remix, adapt, build upon the published works non-commercially, and license the derivative works on different terms, provided the original material is properly cited and the use is non-commercial. Please see: https://creativecommons.org/licenses/by-nc/4.0/
Publisher’s Note:
The opinions, statements, and data contained in article are solely those of the authors and not of Surgery, Gastroenterology and Oncology journal or the editors. Publisher and the editors disclaim responsibility for any damage resulting from any ideas, instructions, methods, or products referred to in the content.
 IASGO Society News
IASGO Society News