Surgery, Gastroenterology and Oncology

|
|
Purpose: Many factors, including body composition, can influence the prognosis of colo-rectal cancer (CRC). This study aimed to investigate the effect of sarcopenia on survival in patients subjected to elective resection of colorectal cancer.
Methods: The study included 93 patients with adenocarcinoma of the colon or rectum scheduled for elective resection. Sarcopenia was diagnosed by CT scan measurement of the total cross-sectional area of the psoas muscles (TPA).
Results: Sarcopenia was detected in 29 patients (31.2%). The only factor associated with sarcopenia was tumor size (p < 0.001). Sarcopenia was associated with worse overall survival (OS) (50.4% vs. 86.7% in non-sarcopenic patients, p < 0.001, HR: 5.58, 95%CI: 2.19-14.24). Sarcopenia was associated with worse disease free survival (47.7% vs. 77.1% in non-sarcopenic patients, p = 0.004, HR: 3.37, 95%CI: 1.53-7.43).
Conclusion: Sarcopenia is an independent factor negatively affecting the overall and disease-free survival of patients surgically treated for colorectal cancer.
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer and the second cause of cancer mortalities all over the world (1,2). In Egypt, CRC ranked 8th among commonly diagnosed cancers (3). Surgical resection is the main treatment modality of non-metastatic CRC, based on resecting tumor mass and eliminating draining lymph nodes (4). The prognosis of CRC is influenced by many factors including, tumor stage, potentially curative surgery, grade, histology, and location, among many others (5). Nevertheless, the oncological outcome may also be negatively affected by surgical complications and patient-related factors (6), especially body composition and functional status (7).
Sarcopenia was demonstrated as a risk factor for reduced survival in many cancers (8-10) including CRC (11,12). However, other studies did not demonstrate this relationship (13). Sarcopenia is defined by diminished muscle strength, quantity or quality, and physical performance (14). Moreover, cancer is frequently associated with weight loss and reduction of muscle mass (15).
The goal of this study was to investigate the effect of sarcopenia on survival in patients subjected to elective resection of colorectal cancer.
PATIENTS AND METHODS
This study included a consecutive sample of eligible patients with adenocarcinoma of the colon or rectum scheduled for elective colorectal resection from September 2014 to April 2017. The diagnosis was confirmed upon pathological examination of biopsy specimens. As judged by the relevant clinical specialists, stage 4 cases with resectable liver and lung metastases were included. Informed consent was taken from all cases. The study protocol adheres to the ethical guidelines of the 1975 Declaration of Helsinki. The ethical committee of Cairo University Hsopitals approved the protocol (N-89-2024).
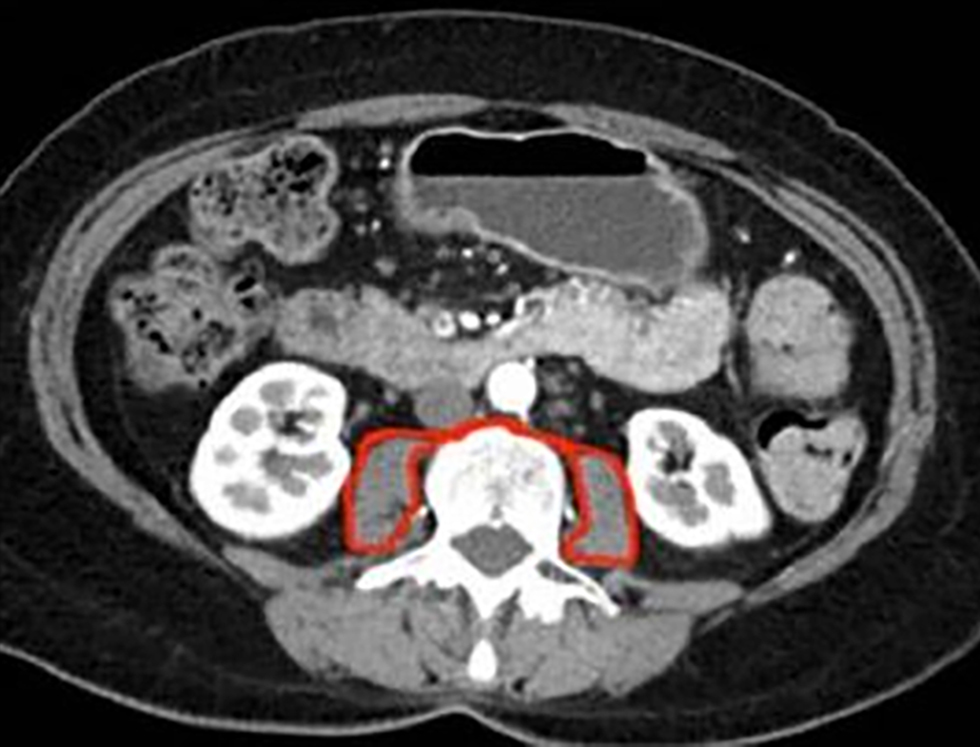
Figure 1 - Total psoas area (TPA) at the level of L3 with both vertebral spines visible
According to our institutional protocol, patients with rectal cancer received neoadjuvant chemo- and radiotherapy with postoperative adjuvant chemo- and radiotherapy as well. On the other hand, stage II, III, and IV colonic cancer received postoperative adjuvant chemotherapy, while some selected cases of stage IV colon cancer received radiotherapy. In patients with stage 0 disease, colonoscopic resection was inadequate due to inadequate surgical margins, so we proceeded to surgery.
Patients were excluded if they received neoadjuvant therapy for colon cancer, had an operation to manage a disease other than colorectal cancer, had widespread unresectable metastatic disease, or had an emergency operation.
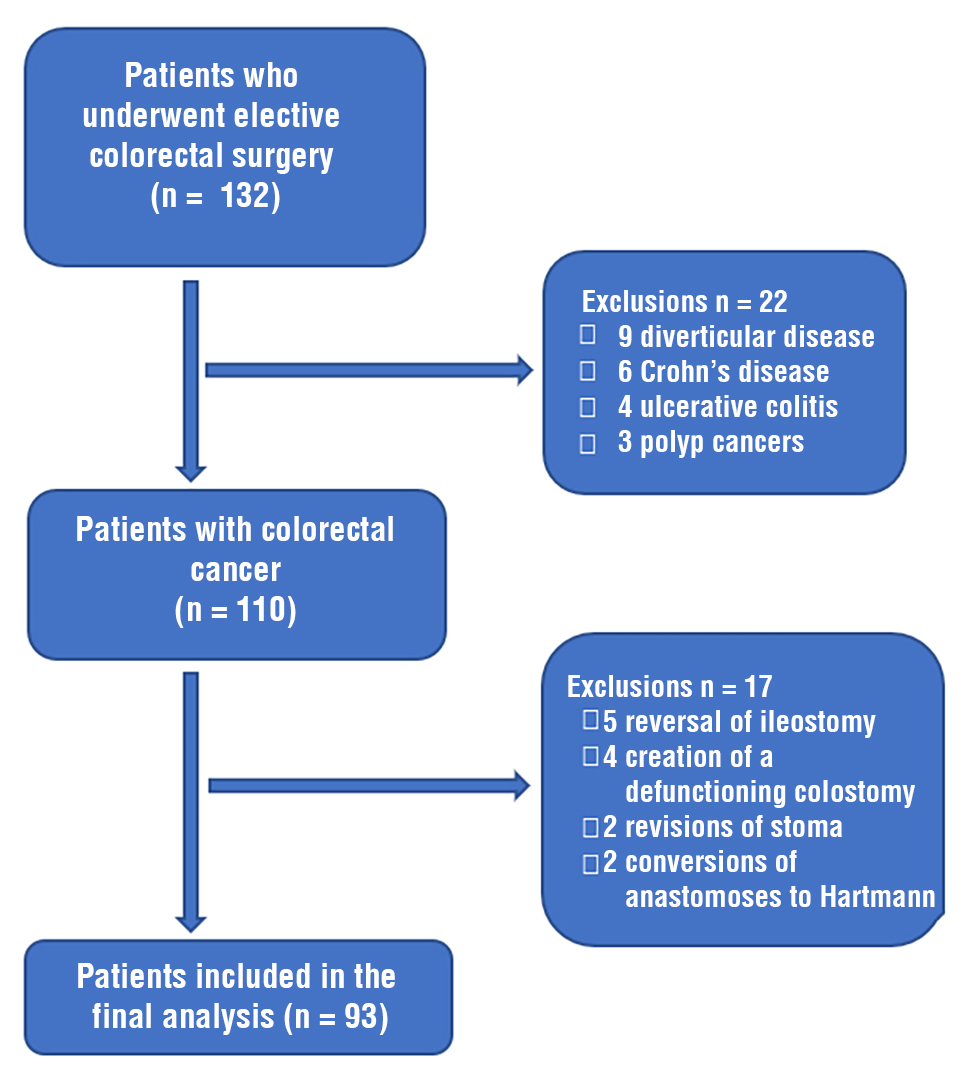
Figure 2
Demographic, anthropometric, and clinical data encompassing height, weight, body mass index (BMI), American Society of Anesthesiologists (ASA) score, operative procedure, American Joint Committee on Cancer (AJCC) TNM stage (16), duration of hospital stay (days), postsurgery complications, and readmission to the hospital within 30 days were registered. All cases were followed up for a minimum of 12 months after resection.
Sarcopenia Measurement by CT Scan
The total cross-sectional area of the psoas muscles (total psoas area, TPA) was assessed employing a manual technique at the level of the L3 vertebra during the preoperative CT scan (17). To confirm standardization, the precise level of assessment was determined as the CT slice in which both L3 transverse processes were most clearly visible. The area was delineated with a free-hand drawing method on Picture Archiving and Communication System (PACS) software (fig. 1). The contour of each psoas muscle was delineated. The area of each was calculated and summated to provide the TPA (mm2). The TPA was then standardized for patient height using the formula: TPA (mm2)/height (m2). This provided the total psoas index (TPI) for each patient.
Table 1 shows the baseline characteristics of the studied group. Eight patients had stage 4 tumors;
For the purpose of this work, the threshold numbers defined for the diagnosis of sarcopenia are the same as those described by Prado et al. in their widely cited research (18); 524 mm2/m2 for males and 385 mm2/m2 for females. All subjects with a TPI below this value were considered sarcopenic. Sixteen scans were randomly chosen and assessed for TPI by blindly competent physicians to ensure technique reliability to estimate inter- and intra-class correlation coefficients (ICCC). The ICCC thresholds for inter and intra-class reliability were 0.93 and 0.98, respectively.
Table 2 - Clinical and Laboratory characteristics of the studied group (n=93)

Statistical Methods
Statistical analysis was done using IBM© SPSS© Statistics version 26 (IBM© Corp., Armonk, NY, USA). Chi-square test was employed to define the relation between qualitative data. For quantitative data, comparison between two arms was made using independent sample t-test or Mann-Whitney test. Survival analysis was done employing Kaplan-Meier method, and contrast between two survival curves was done using log-rank analysis. Multivariate analysis was done using Cox proportional-hazards model for factors affecting survival on univariate analysis. Hazard ratio (HR) with its 95% confidence interval (CI) was used for hazard estimation. A p-value < 0.05 was viewed as significant.
RESULTS
Throughout the research time, 132 patients underwent elective colorectal surgery; 93 (70.4%) were eligible for inclusion in the study (fig. 2).
however, they had resectable lung/liver metastases, so they were operated upon with curative intent. Mucinous adenocarcinoma was the most encountered pathological type, followed by Signet ring cell adeno-carcinoma. Most patients (78.5%) present with stage II or II disease. Surgical resection succeeded in achieving an R0 resection margin in 88.2% of cases. Neoadjuvant chemoradiotherapy and adjuvant radiotherapy were used for patients with rectal cancer only. A single patient with stage IV colon cancer did not receive adjuvant chemotherapy. Table 2 shows the clinical and laboratory characteristics of the studied group.
Sarcopenia was detected in 29 patients (31.2%). Table 3 shows the relationship between sarcopenia diagnosis and patients’ and disease factors. Sarcopenia was not related to the type of cancer, stage, grade, or treatment modalities (table 4). The only factor associated with sarcopenia was tumor size; larger tumors were associated with sarcopenia (p < 0.001). One quarter of the patients developed in-hospital complications in the form of anastomotic leak (n=6), dehydration (n=4), peritoneal infections (n=4), pulmonary embolism (n=3), surgical site infection (n=3), ileus (n=2), chest infection (n=1), and bleeding (n=1). There was no relationship between sarcopenia and in-hospital complications (p = 0.792).
Table 3 - Comparison between patients with and without sarcopenia regarding patients’ and disease characteristics and in-hospital complications

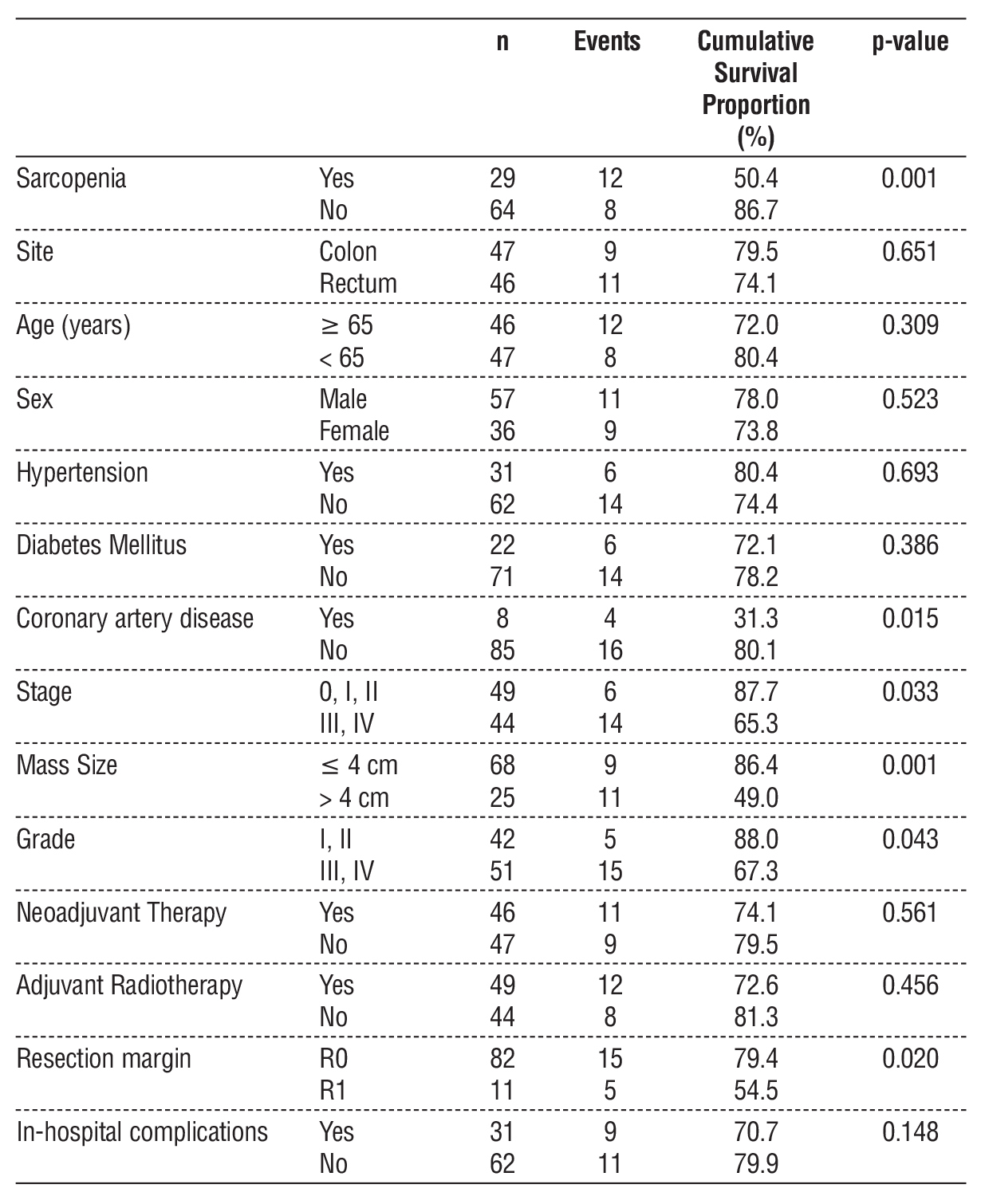
The cumulative overall survival (OS) proportion of the whole group at one year was 76.5%. Table 4 shows factors affecting OS. Sarcopenia was associated with worse survival (50.4% vs. 86.7% in non-sarcopenic patients, p < 0.001). Other factors affecting OS were coronary artery disease (p = 0.015), disease stage (p = 0.033), tumor size (p = 0.001), grade (p = 0.043), and resection margin status (p = 0.020).
Table 4 - Factors associated with overall survival at one year in the studied group
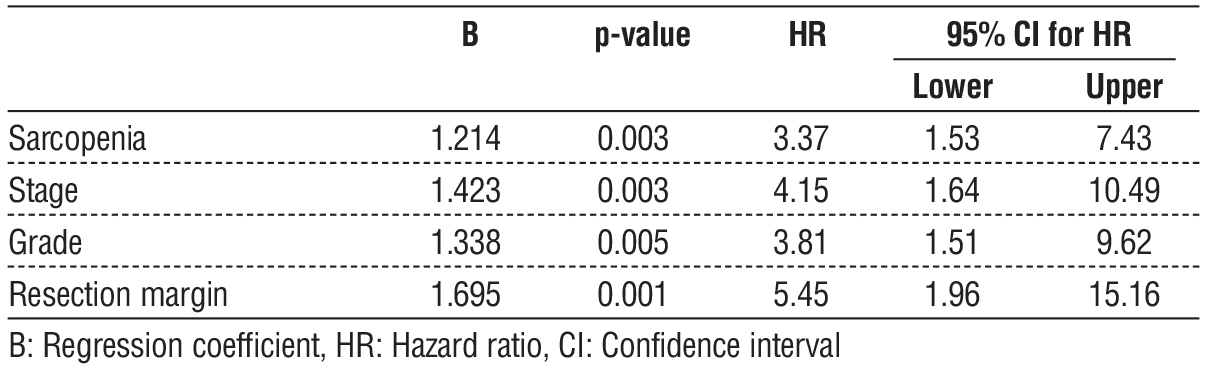
On multivariate analysis, sarcopenia, coronary artery disease, high grade, and positive resection margin were independent factors associated with worse OS (table 5). Sarcopenia carried a nearly 6-fold higher hazard of death in the first year.
Table 5 - Multivariate Cox Proportional Hazard Model for factors affecting overall survival

During the follow-up period, seven patients developed local recurrences. Therefore, the disease-free survival (DFS) was 68.6% at one year. Table 6 shows the factors affecting DFS. Almost the same factors worsened DFS as OS. Sarcopenia was associated with worse DFS (47.7% vs. 77.1% in non-sarcopenic patients, p = 0.004). DFS was worsened by advanced stage (p = 0.016), larger tumors (p = 0.031), higher grade (p = 0.0.31), positive resection margin (p = 0.018), and in-hospital complications (p = 0.001).
Table 6 - Factors associated with recurrence-free survival at two years in the studied group

On multivariate analysis, sarcopenia, advanced stage, high grade, and positive resection margin were independent factors associated with worse DFS (table 7). Sarcopenia carried over a 3-fold higher hazard of recurrence or death at one year.
Table 7 - Multivariate Cox Proportional Hazard Model for factors affecting disease-free survival
DISCUSSION
This study demonstrated a negative impact of sarcopenia on short-term outcomes in patients surgically treated for colorectal cancer. Sarcopenic patients had reduced 1-year overall and disease-free survival. On multivariate analysis, sarcopenia was an independent factor associated with worse OS (HR: 5.6, 95%CI: 2.2-14.2), in addition to coronary artery disease, higher grade, and positive resection margin. Sarcopenia was also an independent predictor of worse DFS (HR: 3.4, 95%CI: 1.5-7.4) combined with advanced stage, high grade, and positive resection margin.
We detected sarcopenia 31.2% of the current series with no significant difference between the colon and rectal cases (p = 0.878). The prevalence of sarcopenia in surgically treated CRC patients varied from 12% to 60% (19–22). Huang et al. (19) reported the lowest prevalence of 11.9% in a cohort of 142 patients with CRC stages I to III. In a group of 259 patients with stage IV CRC, 16% of the patients had sarcopenia (23). Reisinger et al. (24) reported a high prevalence of 47.7% in 310 patients with CRC over 70 years old. In a large cohort of 3262 patients with stage I-III CRC, sarcopenia was highly common (42.4%) (25). In a recent systematic review, the pooled prevalence of sarcopenia was 37% in 18,891 with CRC (26).
In the current series, the only factor associated with sarcopenia was tumor size; larger tumors were more commonly associated with sarcopenia (p < 0.001). No significant relationship was found between sarcopenia and age. In the literature, the results are controversial about this association. Kroenke et al. (25) found that sarcopenia was more common in older patients (>70-80 years) than those < 50 years. Nakanishi et al. (20) reported a higher prevalence of sarcopenia in males and those with low body mass index, but not with old age.
In the present study, sarcopenia did not show a significant association with postoperative complications. This finding differs from what is reported in the most recent systematic review (26). The authors reported a pooled association from 23 studies between sarcopenia and a higher risk of postoperative complications (OR: 1.84; 95%CI: 1.35–2.49). In a recent study, sarcopenia was noticed as a predictor of a higher probability of major complications (p = 0.003) (27). Aro et al. (28) showed an association between sarcopenia and higher rates of pneumonia and cardiorespiratory complication, while there was no difference in other complications.
Sarcopenia was associated with worse OS (50.4% vs. 86.7% in non-sarcopenic patients, p < 0.001) and DFS (47.7% vs. 77.1% in non-sarcopenic patients, p = 0.004) in the present study. In a retrospective study of 220 patients with stage I-III CRC, sarcopenia was associated with a poor outcome. Five-year OS and RFS were significantly shorter in sarcopenic patients (OS, 68 vs. 85%, p = 0.015; RFS, 56 vs. 79 %, p = 0.006;). On multivariate analysis, sarcopenia was an independent predictor of OS and RFS (29). Another study demonstrated significantly lower OS in sarcopenic patients, while the recurrence-free survival (RFS) rate was not different compared to patients with no sarcopenia (30).
A systematic review of 12 studies involving 5337 patients with CRC showed that sarcopenia predicted a decreased OS and DFS (31). A more recent systematic review of 44 studies revealed significantly shorter OS (HR: 1.83; 95% CI: 1.57-2.14) and DFS (HR: 1.55; 95% CI: 1.29-1.88) in sarcopenic as compared with non-sarcopenic patients (26).
Previous studies reported that sarcopenia and systemic inflammation are independent factors for a decreased OS and RFS. Their combination is a predictor of higher risk (32). A more recent study found that sarcopenic obesity and inflammatory status were independent factors affecting OS (30). Sarcopenia combined with inflammation almost doubled the mortality risk of OS in patients with non-metastatic CRC (33). Inflammation was shown to be stage-dependent (28,34). Decreased skeletal muscle density promotes systemic inflammation, which accelerates tumor cell proliferation leading to worse survival (35).
The mechanism of how sarcopenia affects survival of cancer patients remains undetermined. It appears to be multifactorial. Systemic inflammation is known to increase the risk of cancer (36). It was suggested that inflammation is enhanced by muscle breakdown. In CRC, markers of systemic inflammation are correlated with elevated circulating cytokines involved in activating several catabolic pathways. Tumor necrosis factor inhibits skeletal myocyte differentiation and promotes muscle atrophy, and interleukin-6 can reduce muscle protein synthesis. The tumor causes low-grade systemic inflammation that may lead to local inflammation in the muscle. This increases systemic inflammation and muscle degradation (32).
The association of sarcopenia with cancer mortality is suggested to run through its direct and indirect role in cancer management. Sarcopenia is a symbol of cachexia (37,38). It reduces tolerance to anti-cancer treatments (39,40). It was found as an independent factor of cancer treatment toxicity, including chemotherapy targeted therapy (39,41). Reduced muscle mass is shown to be associated with abnormal nutritional status, which is associated with increased mortality (42). Increased mortality may also be due to decreased immune function due to the reduced amino acid substrate required for stress response. Altered voluntary muscles and reduced function may lead to impaired physiologic homeostasis as these muscles are necessary to generate force and facilitate movement (43,44).
The study is not with out limitaions. Firstly, the participation of more male patients than females might affect the study results. Other limitations include being a non-randomized controlled trial. Also, further studies are warranted to elucidate the long-term effect (more than one year) of sarcopenia on survival after resection for colorectal cancer Despite the above, to our knowledge, only a few studies focused on the impact of sarcopenia after elective colorectal cancer surgery. Furthermore, the reasonable sample size of the current study is one of the strengths of this study. This article adds momentum to the growing literature by suggesting that sarcopenia can considerably impact survival rates following resection of colorectal cancer. This work may provide innovative perspectives to classify patient’s pre-operative risk, allowing targeted approaches such as rehabilitation to be adopted to modify sarcopenia and enhance long-term outcomes for these subjects.
CONCLUSIONS
We can conclude that in patients subjected to curative surgical treatment of colorectal cancers, sarcopenia is found in nearly one-third of cases. It had a negative impact on short-term outcomes in terms of one-year survival. Sarcopenic patients had reduced one-year overall and disease-free survival. Sarcopenia was an independent factor associated with worse OS with coronary artery disease, higher grade, and positive resection margin. It was also an independent predictor of worse disease-free survival combined with advanced stage, high grade, and positive resection margin. It is recommended to investigate the effect of sarcopenia on the long-term outcomes of these patients.
Conflict of Interest
No interests to declare
Funding
No funding was received.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, (Salman Ahmed), upon reasonable request.
REFERENCES
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
2. Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017; 67:177–93.
3. Ibrahim AS, Khaled HM, Mikhail NN, Baraka H, Kamel H. Cancer Incidence in Egypt: Results of the National Population-Based Cancer Registry Program. Journal of Cancer Epidemiology. Hindawi; 2014; 2014:e437971.
4. Salibasic M, Pusina S, Bicakcic E, Pasic A, Gavric I, Kulovic E, et al. Colorectal Cancer Surgical Treatment, our Experience. Med Arch. 2019;73:412–4.
5. Compton CC, Fielding LP, Burgart LJ, Conley B, Cooper HS, Hamilton SR, et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:979–94.
6. Sun G, Li Y, Peng Y, Lu D, Zhang F, Cui X, et al. Can sarcopenia be a predictor of prognosis for patients with non-metastatic colorectal cancer? A systematic review and meta-analysis. Int J Colorectal Dis. 2018;33:1419–27.
7. Vergara-Fernandez O, Trejo-Avila M, Salgado-Nesme N. Sarcopenia in patients with colorectal cancer: A comprehensive review. World J Clin Cases. 2020;8:1188–202.
8. Kawaguchi Y, Hanaoka J, Ohshio Y, Okamoto K, Kaku R, Hayashi K, et al. sarcopenia predicts poor postoperative outcome in elderly patients with lung cancer. Gen Thorac Cardiovasc Surg. 2019;67: 949–54.
9. Rier HN, Jager A, Sleijfer S, van Rosmalen J, Kock MCJM, Levin M-D. Low muscle attenuation is a prognostic factor for survival in metastatic breast cancer patients treated with first line palliative chemotherapy. The Breast. 2017;31:9–15.
10. Dijk DPJ van, Bakens MJAM, Coolsen MME, Rensen SS, Dam RM van, Bours MJL, et al. Low skeletal muscle radiation attenuation and visceral adiposity are associated with overall survival and surgical site infections in patients with pancreatic cancer. Journal of Cachexia, Sarcopenia and Muscle. 2017;8:317–26.
11. Hopkins JJ, Reif RL, Bigam DL, Baracos VE, Eurich DT, Sawyer MB. The Impact of Muscle and Adipose Tissue on Long-term Survival in Patients With Stage I to III Colorectal Cancer. Dis Colon Rectum. 2019;62:549–60.
12. Miyamoto Y, Baba Y, Sakamoto Y, Ohuchi M, Tokunaga R, Kurashige J, et al. sarcopenia is a Negative Prognostic Factor After Curative Resection of Colorectal Cancer. Ann Surg Oncol. 2015;22:2663–8.
13. van Vugt JLA, Coebergh van den Braak RRJ, Lalmahomed ZS, Vrijland WW, Dekker JWT, Zimmerman DDE, et al. impact of low skeletal muscle mass and density on short and long-term outcome after resection of stage I-III colorectal cancer. Eur J Surg Oncol. 2018;44:1354–60.
14. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age and Ageing. 2019;48:16–31.
15. Aversa Z, Costelli P, Muscaritoli M. Cancer-induced muscle wasting: latest findings in prevention and treatment. Ther Adv Med Oncol. 2017;9:369–82.
16. Colorectal Cancer Stages | Rectal Cancer Staging | Colon Cancer Staging. Available from: https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/staged.html
17. Jones KI, Doleman B, Scott S, Lund JN, Williams JP. Simple psoas cross-sectional area measurement is a quick and easy method to assess sarcopenia and predicts major surgical complications. Colorectal Dis. 2015;17:O20-26.
18. Prado CMM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–35.
19. Huang D-D, Wang S-L, Zhuang C-L, Zheng B-S, Lu J-X, Chen F-F, et al. sarcopenia, as defined by low muscle mass, strength and physical performance, predicts complications after surgery for colorectal cancer. Colorectal Disease. 2015;17:O256–64.
20. Nakanishi R, Oki E, Sasaki S, Hirose K, Jogo T, Edahiro K, et al. Sarcopenia is an independent predictor of complications after colo-rectal cancer surgery. Surg Today. 2018;48:151–7.
21. Dolan DR, Knight KA, Maguire S, Moug SJ. The relationship between sarcopenia and survival at 1 year in patients having elective colorectal cancer surgery. Tech Coloproctol. 2019;23:877–85.
22. Hopkins JJ, Reif RL, Bigam DL, Baracos VE, Eurich DT, Sawyer MB. The Impact of Muscle and Adipose Tissue on Long-term Survival in Patients With Stage I to III Colorectal Cancer. Dis Colon Rectum. 2019;62:549–60.
23. Peng PD, van Vledder MG, Tsai S, de Jong MC, Makary M, Ng J, et al. sarcopenia negatively impacts short-term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. HPB (Oxford). 2011;13:439–46.
24. Reisinger KW, van Vugt JLA, Tegels JJW, Snijders C, Hulsewé KWE, Hoofwijk AGM, et al. Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann Surg. 2015;261: 345–52.
25. Kroenke CH, Prado CM, Meyerhardt JA, Weltzien EK, Xiao J, Cespedes Feliciano EM, et al. Muscle radiodensity and mortality in patients with colorectal cancer. Cancer. 2018;124:3008–15.
26. Trejo-Avila M, Bozada-Gutiérrez K, Valenzuela-Salazar C, Herrera-Esquivel J, Moreno-Portillo M. Sarcopenia predicts worse post-operative outcomes and decreased survival rates in patients with colorectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 2021;36:1077–96.
27. Pereira M, Pereira A, Silva P, Costa C, Martins SF. Sarcopenia as a Risk Factor of Morbimortality in Colorectal Cancer Surgery. Gastrointestinal Disorders. Multidisciplinary Digital Publishing Institute; 2020;2:107–17.
28. Aro R, Mäkäräinen-Uhlbäck E, Ämmälä N, Rautio T, Ohtonen P, Saarnio J, et al. The impact of sarcopenia and myosteatosis on postoperative outcomes and 5-year survival in curatively operated colorectal cancer patients - A retrospective register study. Eur J Surg Oncol. 2020;46:1656–62.
29. Miyamoto Y, Baba Y, Sakamoto Y, Ohuchi M, Tokunaga R, Kurashige J, et al. sarcopenia is a Negative Prognostic Factor After Curative Resection of Colorectal Cancer. Ann Surg Oncol. 2015;22:2663–8.
30. Han JS, Ryu H, Park IJ, Kim KW, Shin Y, Kim SO, et al. Association of Body Composition with Long-Term Survival in Non-metastatic Rectal Cancer Patients. Cancer Res Treat. 2020;52:563–72.
31. Sun G, Li Y, Peng Y, Lu D, Zhang F, Cui X, et al. Can sarcopenia be a predictor of prognosis for patients with non-metastatic colorectal cancer? A systematic review and meta-analysis. Int J Colorectal Dis. 2018;33:1419–27.
32. Feliciano EMC, Kroenke CH, Meyerhardt JA, Prado CM, Bradshaw PT, Kwan ML, et al. Association of Systemic Inflammation and Sarcopenia With Survival in Non-metastatic Colorectal Cancer: Results From the C SCANS Study. JAMA Oncol. 2017;3:e172319.
33. Cespedes Feliciano EM, Avrutin E, Caan BJ, Boroian A, Mourtzakis M. Screening for low muscularity in colorectal cancer patients: a valid, clinic-friendly approach that predicts mortality. J Cachexia Sarcopenia Muscle. 2018;9:898–908.
34. Rasic I, Radovic S, Aksamija and. Relationship Between Chronic Inflammation and the Stage and Histopathological Size of Colorectal Carcinoma. Med Arh. 2016;70:104.
35. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420: 860–7.
36. Korniluk A, Koper O, Kemona H, Dymicka-Piekarska V. From inflammation to cancer. Ir J Med Sci. 2017;186:57–62.
37. Hilmi M, Jouinot A, Burns R, Pigneur F, Mounier R, Gondin J, et al. Body composition and sarcopenia: The next-generation of personalized oncology and pharmacology? Pharmacol Ther. 2019; 196:135–59.
38. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–95.
39. Antoun S, Baracos VE, Birdsell L, Escudier B, Sawyer MB. Low body mass index and sarcopenia associated with dose-limiting toxicity of sorafenib in patients with renal cell carcinoma. Ann Oncol. 2010;21:1594–8.
40. Prado CMM, Baracos VE, McCargar LJ, Mourtzakis M, Mulder KE, Reiman T, et al. Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Cancer Res. 2007; 13:3264–8.
41. Go S-I, Park MJ, Song H-N, Kim H-G, Kang MH, Lee HR, et al. Prognostic impact of sarcopenia in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Cachexia Sarcopenia Muscle. 2016;7:567–76.
42. Arends J, Baracos V, Bertz H, Bozzetti F, Calder PC, Deutz NEP, et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr. 2017;36:1187–96.
43. Kamei Y, Hatazawa Y, Uchitomi R, Yoshimura R, Miura S. Regulation of Skeletal Muscle Function by Amino Acids. Nutrients. 2020;12: E261.
44. Tsai S. Importance of Lean Body Mass in the Oncologic Patient. Nutrition in Clinical Practice. 2012;27:593–8.
Full Text Sources:
Abstract:
Views: 1212

For Authors
Journal Subscriptions

Jun 2025
Supplements
Instructions for authors
Online submission
Contact
e-ISSN: 2601 - 1700 (online)
ISSN-L: 2559 - 723X
Journal Abbreviation: Surg. Gastroenterol. Oncol.
Surgery, Gastroenterology and Oncology (SGO) is indexed in:
- SCOPUS
- EBSCO
- DOI/Crossref
- Google Scholar
- SCImago
- Harvard Library
- Open Academic Journals Index (OAJI)
Surgery, Gastroenterology and Oncology (SGO) is an open-access, peer-reviewed online journal published by Celsius Publishing House. The journal allows readers to read, download, copy, distribute, print, search, or link to the full text of its articles.
Time to first editorial decision: 25 days
Rejection rate: 61%
CiteScore: 0.2

Meetings and Courses in 2025
Meetings and Courses in 2024
Meetings and Courses in 2023
Meetings and Courses in 2022
Meetings and Courses in 2021
Meetings and Courses in 2020
Meetings and Courses in 2019
Verona expert meeting 2019

Surgery, Gastroenterology and Oncology applies the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits readers to copy and redistribute the material in any medium or format, remix, adapt, build upon the published works non-commercially, and license the derivative works on different terms, provided the original material is properly cited and the use is non-commercial. Please see: https://creativecommons.org/licenses/by-nc/4.0/
Publisher’s Note:
The opinions, statements, and data contained in article are solely those of the authors and not of Surgery, Gastroenterology and Oncology journal or the editors. Publisher and the editors disclaim responsibility for any damage resulting from any ideas, instructions, methods, or products referred to in the content.
 IASGO Society News
IASGO Society News